Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 02, 2023 2 years, 3 weeks, 6 days, 19 hours, 37 minutes ago
HIV News: In a significant leap forward in the ongoing battle against HIV, researchers at the Yale School of Medicine have recently revealed a detailed understanding of the intricate processes governing the early stages of HIV infection. Through the application of cutting-edge cryogenic electron tomography (cryo-ET), the study covered in this
HIV News report, not only unraveled the complex interplay between the human immunodeficiency virus (HIV) and T cells but also illuminated potential therapeutic avenues. The insights garnered into the binding mechanisms may herald a transformative era in treatment strategies, fostering the development of targeted medications capable of inhibiting specific HIV conformations.
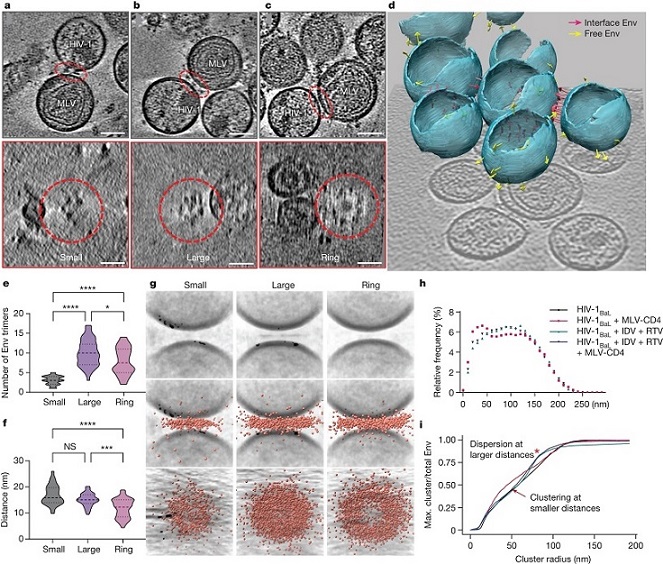 a–c, Representative tomograms of small (a), large (b) and ring (c) Env cluster formations. The interfaces are indicated by red dashed ovals. Bottom, top-down views of each interface, revealing Env clustering. Scale bars, 50 nm. d, Three-dimensional representation of viral particles in a representative tomogram. The pink arrows represent Env trimers at membrane–membrane interfaces. The yellow arrows represent free Env trimers on the surface of HIV-1BaL. e, The number of Env trimers present at interfaces in each clustering pattern. Mean ± s.d. = 3.0 ± 1.1 (small), 10.0 ± 3.4 (large) and 7.9 ± 3.4 (ring). *P = 0.0478, ****P < 0.0001. f, The distance between the membranes at the interfaces for each clustering pattern. Mean ± s.d. = 16.9 ± 3.8 nm (small), 15.1 ± 2.4 nm (large) and 11.9 ± 3.6 nm (ring). NS, P = 0.4178; ***P = 0.0002, ****P < 0.0001. g, Subtomogram-averaged interfaces from small, large and ring clusters. Individual subtomograms of the membrane–membrane interfaces from each class were aligned, and the coordinates of Env–CD4 complexes were overlaid and displayed as side and top-down views (middle and bottom, respectively). h,i, Clustering analysis of Env trimers on the surface of mature (black) and immature HIV-1BaL particles alone (green, prepared by treating virus-producing cells with the protease inhibitors indinavir (IDV) and ritonavir (RTV)), and mature (red) and immature (purple) HIV-1BaL particles mixed with MLV-CD4 VLPs. h, Histogram profile of the arc distances between Env trimers on the surface of particles. i, Multidistance spacial cluster analysis of the ratio of the largest number of Env trimers with increasing cluster radii to the total Env trimers on each viral particle. Env clustering (arrow) and dispersion (red asterisk) are indicated. Max., maximum.
Current Landscape of HIV Treatment
a–c, Representative tomograms of small (a), large (b) and ring (c) Env cluster formations. The interfaces are indicated by red dashed ovals. Bottom, top-down views of each interface, revealing Env clustering. Scale bars, 50 nm. d, Three-dimensional representation of viral particles in a representative tomogram. The pink arrows represent Env trimers at membrane–membrane interfaces. The yellow arrows represent free Env trimers on the surface of HIV-1BaL. e, The number of Env trimers present at interfaces in each clustering pattern. Mean ± s.d. = 3.0 ± 1.1 (small), 10.0 ± 3.4 (large) and 7.9 ± 3.4 (ring). *P = 0.0478, ****P < 0.0001. f, The distance between the membranes at the interfaces for each clustering pattern. Mean ± s.d. = 16.9 ± 3.8 nm (small), 15.1 ± 2.4 nm (large) and 11.9 ± 3.6 nm (ring). NS, P = 0.4178; ***P = 0.0002, ****P < 0.0001. g, Subtomogram-averaged interfaces from small, large and ring clusters. Individual subtomograms of the membrane–membrane interfaces from each class were aligned, and the coordinates of Env–CD4 complexes were overlaid and displayed as side and top-down views (middle and bottom, respectively). h,i, Clustering analysis of Env trimers on the surface of mature (black) and immature HIV-1BaL particles alone (green, prepared by treating virus-producing cells with the protease inhibitors indinavir (IDV) and ritonavir (RTV)), and mature (red) and immature (purple) HIV-1BaL particles mixed with MLV-CD4 VLPs. h, Histogram profile of the arc distances between Env trimers on the surface of particles. i, Multidistance spacial cluster analysis of the ratio of the largest number of Env trimers with increasing cluster radii to the total Env trimers on each viral particle. Env clustering (arrow) and dispersion (red asterisk) are indicated. Max., maximum.
Current Landscape of HIV Treatment
Despite decades of extensive research, HIV remains a formidable global health challenge, with the absence of a vaccine or definitive cure. Existing treatments predominantly rely on antiretroviral therapy (ART) to suppress viral replication. However, these therapeutic interventions demand a lifelong commitment, and
their efficacy can diminish over time for certain patient populations. The recent study conducted at Yale sought to deepen the understanding of the initial stages of HIV infection, particularly the binding process to T cells, with the ultimate goal of identifying vulnerabilities that could be exploited for more effective therapeutic interventions.
Cryo-ET Unveils the Structural Insights
Under the leadership of Dr Walther Mothes, the Paul B. Beeson Professor of Medicine at Yale School of Medicine, the research team harnessed the power of cryo-ET to visualize the interaction between HIV-1 and virus-like particles (VLPs) carrying CD4 receptors. These VLPs were designed as mimics of the natural interaction between HIV and T cells, providing an unprecedented close-up view of the binding process at a structural level.
The observations made during the study were remarkably insightful. The team observed the formation of discrete clusters and rings as HIV-1 and VLPs came into contact. The crucial aspect distinguishing different binding stages was the distance between the membranes: when farther apart, HIV-1 bound to one CD4 receptor, and as they approached, it sequentially engaged with a second and third CD4 receptor.
Deciphering the Structural Dynamics: Implications and Applications
Dr Mothes highlighted the transformative nature of the study, underscoring its ability to provide unprecedented insights into the very early stages of HIV infection. Understanding the sequential interactions leading to membrane fusion with T cells now opens avenues for the development of inhibitory medications that can target specific HIV conformations. This precision-targeted approach holds immense promise for a more nuanced and effective strategy in HIV treatment.
Therapeutic Implications for HIV and Beyond
The ramifications of the study extend beyond the realm of HIV, potentially influencing the approach to other viral infections. The overarching goal is to develop inhibitors that precisely target intermediate HIV conformations, effectively preventing the virus from infiltrating host cells. In essence, this targeted strategy can be likened to intercepting rogue viruses without causing collateral damage to other vital molecules in the body.
Dr Wenwei Li, the first author of the study and an associate research scientist in the Mothes Laboratory, offered a pertinent analogy. He compared current HIV drugs to blocking specific lanes to halt the spread of the virus, affecting other molecules like cars in traffic. The aspiration is to gain a profound understanding of the distinctive characteristics of viruses - color, size, and shape - enabling the development of drugs that specifically target them. This signifies a paradigm shift towards precision medicine in the context of infectious diseases.
Looking Forward: The Unexplored Second Act
While the recent study delved deeply into the initial step of HIV infection - the binding to T cells - it is essential to acknowledge the existence of a crucial second step in this intricate process: the fusion of membranes after the virus binds to a host cell. Dr Li emphasized the significance of this second act, indicating that while the current study observed the first step, the research team is eager to explore the intricacies of the second step in forthcoming research endeavors.
Beyond HIV: Applying Techniques to Understand COVID-19
The innovative techniques deployed in the study may have broader applications beyond HIV. The research team at Yale is poised to leverage their methodologies to gain deeper insights into the infection process of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19. This interdisciplinary approach holds the potential to contribute to the development of more effective drugs for combating the ongoing global pandemic.
The study, provides intricate structural insights into the interaction between HIV-1 envelope glycoprotein (Env) and the CD4 receptor. Cryo-ET enabled researchers to directly visualize these interactions in native membranes, offering a high-resolution understanding of the binding process.
The Complexity of HIV-1 Entry
The initiation of HIV-1 infection involves the trimeric HIV-1 Env binding to the CD4 receptor on the surface of target cells. This binding induces conformational changes in the Env glycoprotein, enabling subsequent engagement with co-receptors CCR5 or CXCR4. The recent study focused on understanding the binding process in native membranes, a relatively unexplored territory compared to previous studies employing soluble receptors.
Visualizing the Interaction: Cryo-ET and HIV-1BaL Particles
Cryo-ET emerged as a pivotal technology in characterizing membrane-embedded HIV-1 Env–CD4 interactions using HIV-1BaL particles. These particles, derived from chronically infected SUP-T1 T cells, served as a model system with a high density of Env trimers. The researchers observed membrane–membrane interfaces between HIV-1BaL particles and plasma membrane blebs, providing a detailed glimpse into the intricate interplay of HIV and T cells at the molecular level.
Env Clustering and CD4-Dependent Organization
The cryo-ET tomograms unveiled distinct patterns of Env clustering and ring formation at membrane–membrane interfaces. Notably, the presence of CD4 correlated with increased Env clustering, suggesting a CD4-dependent organization of Env at these interfaces. This finding underscores the nuanced nature of the interaction and highlights potential points of intervention for therapeutic development.
Capsid Maturation Dependency
The study also delved into the role of capsid maturation in CD4-induced Env clustering. Capsid maturation, involving the cleavage of the matrix protein, facilitates Env mobility on the viral particle surface. The researchers discovered that CD4-induced clustering was dependent on capsid maturation, emphasizing the dynamic interplay between viral components during the early stages of infection.
Env Binding Dynamics: Unraveling the Complexity
Subtomogram averaging and classification techniques revealed a dynamic process of Env binding to CD4 receptors. When membranes were further apart, Env engaged a single CD4 molecule. As the membranes approached each other, Env sequentially bound to two or three CD4 molecules. The structural heterogeneity in CD4 binding was evident, with one CD4 molecule exhibiting denser density than the others in the trimer.
Asymmetric Env Trimers: Insights into Intermediate States
The cryo-ET density maps provided high-resolution images of asymmetric HIV-1 Env trimers bound to one, two, and three CD4 molecules. These structures represented detectable intermediates during virus binding to membranes. The V1V2 loops projected outward in CD4-bound protomers, while CD4-free protomers showed a more compact conformation. These asymmetric structures offer crucial insights into the intermediate states of Env trimers during the initial stages of viral entry.
Implications for Therapeutic Development
The study's findings lay the groundwork for innovative therapeutic interventions, focusing on inhibiting specific conformations rather than entire viral pathways. Targeting the dynamic intermediate states observed during CD4 engagement provides a more nuanced approach, minimizing potential side effects associated with broader inhibition strategies. The ultimate aim is to develop precision medications that selectively interfere with the key steps of HIV entry into host cells.
The Road Ahead: Unraveling the Second Act
As researchers celebrate the groundbreaking revelations into the first act of HIV-1 infection - the binding process - they acknowledge the unexplored territory of the second act: membrane fusion. While the recent study provided unprecedented insights into the initial step, the intricate dynamics of membrane fusion following CD4 engagement remain a focal point for future investigations. Elucidating this second act holds the key to a comprehensive understanding of the entire viral entry process.
Broader Implications: A Paradigm Shift in Antiviral Drug Development
The significance of the study extends beyond HIV research, signaling a potential paradigm shift in antiviral drug development. The tailored approach of inhibiting specific conformations rather than entire viral pathways aligns with the principles of precision medicine. This approach not only enhances treatment efficacy but also minimizes the risk of unintended side effects associated with conventional broad-spectrum antiviral medications.
Conclusion: A New Frontier in Infectious Disease Research
Yale's groundbreaking study marks a significant stride forward in unraveling the intricate molecular choreography of HIV-1 entry into T cells. The marriage of cutting-edge cryo-ET technology and meticulous research has provided a roadmap for the development of targeted therapeutics. As researchers set their sights on exploring the second act of HIV-1 infection - membrane fusion - the anticipation is high for further breakthroughs that could catalyze innovative treatments not only for HIV but also for a broader spectrum of infectious diseases, including the ongoing battle against COVID-19. The intricate details of viral entry may soon be deciphered, ushering in a new frontier in the relentless pursuit of effective interventions against infectious diseases on a global scale.
The study findings were published in the peer reviewed journal: Nature
https://www.nature.com/articles/s41586-023-06762-6
For the latest
HIV News, keep on logging to Thailand Medical News.
