Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 28, 2024 5 months, 2 weeks, 6 days, 16 hours, 38 minutes ago
Medical News: Calcium Signals in Fibroblasts: A Path to Understanding Heart and Lung Fibrosis
Researchers at the University of Medicine and Pharmacy "Carol Davila" in Romania and the University of Bucharest are uncovering how calcium signaling in specialized cells, called fibroblasts, plays a crucial role in the development of fibrosis - a process that leads to scarring and stiffening of tissues like the heart and lungs. Their findings could open new doors for treatments aimed at managing or even preventing fibrosis.
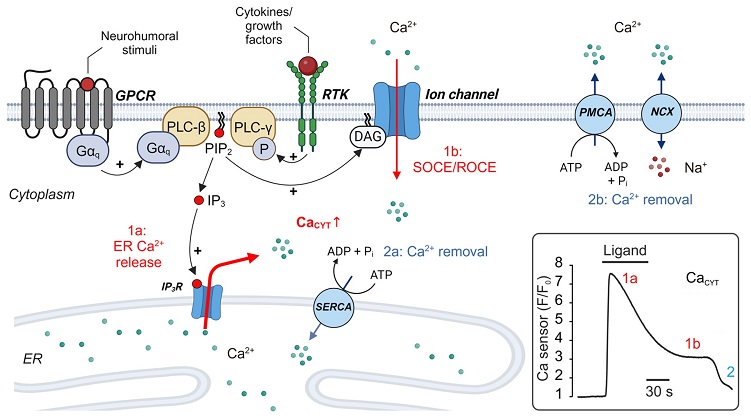 Regulation of cytoplasmic calcium (CaCYT) in fibroblasts. CaCYT increases following stimulation of plasmalemmal receptors and activation of PLC, which triggers Ca2+ release from the ER (1a) and subsequent Ca2+ influx via Ca2+-permeable ion channels (1b). Ca2+ events are terminated by Ca2+-dependent closure of IP3R at high CaCYT levels and resting Ca2+ levels are re-established by Ca2+ transport processes that pump Ca2+ from the cytoplasm into the ER (2a) or extrude Ca2+ to the extracellular space (2b). Inset: simplified time course of intracellular Ca2+ changes in non-excitable cells induced by IP3R-mediated Ca2+ release and SOCE. Abbreviations: ADP: adenosine diphosphate. ATP: adenosine triphosphate. DAG: diacylglycerol. ER: endoplasmic reticulum. Gαq: G alpha q subunit of a heterotrimeric G protein (the βγ-dimer is not displayed for simplicity). GPCR: G protein-coupled receptor. IP3: inositol trisphosphate. PIP2: phosphatidylinositol bisphosphate. PLC: phospholipase C. NCX: Na+/Ca2+ exchanger. PMCA: plasmalemmal Ca2+-ATPase. SERCA: sarcoplasmic/endoplasmic Ca2+-ATPase. RTK: receptor tyrosine kinase. SOCE: store-operated Ca2+ entry. ROCE: receptor-operated Ca2+ entry.
Understanding Fibroblasts and Their Role in Healing
Regulation of cytoplasmic calcium (CaCYT) in fibroblasts. CaCYT increases following stimulation of plasmalemmal receptors and activation of PLC, which triggers Ca2+ release from the ER (1a) and subsequent Ca2+ influx via Ca2+-permeable ion channels (1b). Ca2+ events are terminated by Ca2+-dependent closure of IP3R at high CaCYT levels and resting Ca2+ levels are re-established by Ca2+ transport processes that pump Ca2+ from the cytoplasm into the ER (2a) or extrude Ca2+ to the extracellular space (2b). Inset: simplified time course of intracellular Ca2+ changes in non-excitable cells induced by IP3R-mediated Ca2+ release and SOCE. Abbreviations: ADP: adenosine diphosphate. ATP: adenosine triphosphate. DAG: diacylglycerol. ER: endoplasmic reticulum. Gαq: G alpha q subunit of a heterotrimeric G protein (the βγ-dimer is not displayed for simplicity). GPCR: G protein-coupled receptor. IP3: inositol trisphosphate. PIP2: phosphatidylinositol bisphosphate. PLC: phospholipase C. NCX: Na+/Ca2+ exchanger. PMCA: plasmalemmal Ca2+-ATPase. SERCA: sarcoplasmic/endoplasmic Ca2+-ATPase. RTK: receptor tyrosine kinase. SOCE: store-operated Ca2+ entry. ROCE: receptor-operated Ca2+ entry.
Understanding Fibroblasts and Their Role in Healing
Fibroblasts are cells that contribute to the healing and repair of tissue after injury. In normal circumstances, fibroblasts activate, migrate to the site of injury, and transform into myofibroblasts, which secrete proteins like collagen to build the tissue framework needed for healing. Once the repair is complete, these myofibroblasts typically undergo apoptosis (a form of cell death), leaving the tissue restored.
However, in certain organs such as the heart, fibroblasts often remain active and sensitive to signals that keep them working past the point of healing. This extended activity can lead to fibrosis, a buildup of connective tissue that stiffens the organ. When fibrosis occurs in the heart or lungs, it can severely impact function, as the heart may struggle to pump blood, and lungs may find it difficult to expand and contract fully.
Calcium Signaling: A Central Role in Fibroblast Activation
This
Medical News report explores recent research revealing the role of calcium (Ca²⁺) signals in the activation of fibroblasts. Calcium signals are essential for regulating fibroblast behavior, from proliferation to migration and collagen production. Understanding the pathways that control calcium signals could offer a breakthrough in managing fibrosis.
In most fibroblasts, calcium signal
s originate inside the cell and are regulated by IP3 receptors, a group of proteins that allow the release of calcium stored in the cell’s endoplasmic reticulum (ER). Once calcium is released, it flows into the cytoplasm, where it triggers other cellular processes.
Another key pathway involves ion channels like TRP and SOCE channels, which allow calcium to flow into the cell from the outside environment, maintaining a steady supply to activate various cellular responses. For example, in heart and lung fibroblasts, SOCE channels are particularly important because they help maintain calcium levels necessary for myofibroblast transformation - a key step in fibrosis.
Pathways in Heart Fibrosis
In the heart, fibrosis often occurs after tissue damage, such as a heart attack. When fibroblasts receive certain signals like Angiotensin II, they respond by transforming into myofibroblasts that build scar tissue. This transformation is facilitated by calcium channels such as TRPC3 and TRPC6, which play a critical role in activating transcription factors, proteins that regulate gene expression.
The study also examined other calcium channels such as the Na⁺/Ca²⁺ exchanger (NCX), which help restore calcium levels in the cell after a signaling event. These channels become particularly active in heart fibroblasts, helping sustain the fibroblast’s function as they produce more collagen and fibronectin, proteins essential to the structural stability of heart tissue.
Calcium Signaling in Lung Fibrosis
Calcium signaling pathways also influence fibrosis in the lungs, where inflammation often triggers fibroblast activation. One pathway involves the TRPV4 channel, which senses changes in pressure within the lungs and allows calcium into the fibroblasts. Activated by transforming growth factor beta (TGF-β), a protein linked to fibrosis, TRPV4 opens up, triggering calcium influx that leads to the production of collagen and other extracellular matrix components.
The study revealed that during fibrosis, fibroblasts in the lungs experience a continuous influx of calcium, activating various transcription factors and signaling proteins that help sustain the fibrotic process. By inhibiting TRP channels in animal models, researchers found they could prevent excessive fibroblast activation and reduce fibrosis severity.
Nuclear Calcium: Directing Gene Expression
Calcium also plays a direct role in gene expression within the cell nucleus. Through three different mechanisms, calcium reaches the nucleus where it activates pathways that control genes essential for fibroblast behavior. Nuclear calcium interacts with proteins like calmodulin, which then activate transcription factors such as nuclear factor of activated T cells (NFAT). This factor is particularly important for heart fibroblasts, as it regulates the genes involved in collagen production.
Another pathway activates the protein CREB, a transcription factor that regulates genes critical to fibroblast proliferation. Researchers discovered that blocking calcium’s influence in the nucleus could prevent excessive gene activation and limit fibrosis in both heart and lung fibroblasts.
Future Directions and Therapeutic Implications
The study from Romania’s University of Medicine and Pharmacy "Carol Davila" and the University of Bucharest highlights potential therapeutic targets within calcium signaling pathways. By understanding how these pathways control fibroblast activation and myofibroblast transformation, scientists could develop drugs to selectively inhibit these processes, reducing or preventing fibrosis.
Targeting specific calcium channels could offer a pathway to limiting fibrosis without disrupting essential cell functions. For instance, therapies aimed at blocking TRP channels or SOCE pathways could stop excessive fibroblast activation in the heart and lungs. Likewise, nuclear calcium pathways present promising areas for intervention, especially in reducing collagen production and limiting scar tissue formation.
Conclusions
This research suggests that controlling calcium signaling in fibroblasts could provide a therapeutic advantage in treating or even preventing fibrosis. By identifying key signaling pathways, the study offers insights into how fibroblasts contribute to scarring and tissue stiffness in the heart and lungs. Effective therapies targeting these pathways could help manage conditions like heart failure and pulmonary fibrosis, where tissue stiffness poses serious health risks.
Importantly, this study demonstrates the intricate relationship between calcium signals and fibroblast activity, highlighting how nuanced these cellular interactions are.
The study findings were published in the peer-reviewed journal: Biomolecules.
https://www.mdpi.com/2218-273X/14/11/1365
For the latest on Fibrosis, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-can-lead-to-heart-fibrosis
https://www.thailandmedical.news/news/covid-19-is-not-mild-as-most-will-develop-lung-fibrosis-10-percent-of-all-lung-transplants-in-u-s-now-go-to-post-covid-patients
