Charles Tee Fact checked by:Thailand Medical News Team Sep 05, 2024 7 months, 3 weeks, 8 hours, 10 minutes ago
Medical News: Hypernatremia, a condition characterized by elevated sodium levels in the bloodstream, poses serious risks to brain health. In a groundbreaking study led by researchers from Fujita Health University in Japan, new insights were revealed into how hyperosmotic stress, driven by high sodium levels, affects microglial cells, which are crucial to maintaining brain function. Microglia serve as the brain's immune cells, responding to external threats and ensuring neural stability. When exposed to hypernatremia, these cells react in complex ways that can lead to harmful consequences. This
Medical News report dives into the mechanisms behind these cellular responses and explores potential therapeutic avenues for mitigating the effects of hypernatremia on the brain.
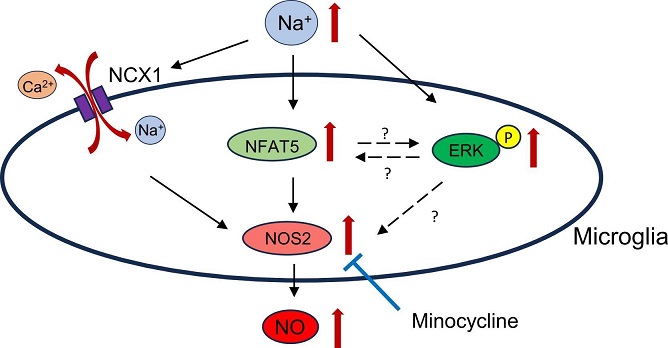 Schematic diagram of the effect of hypernatremia on microglia
Schematic diagram of the effect of hypernatremia on microglia
The research team, led by Professor Yoshihisa Sugimura from the Department of Endocrinology, Diabetes, and Metabolism at Fujita Health University-Japan, collaborated with fellow researchers Sachiho Fuse, Haruki Fujisawa, and Atsushi Suzuki. Their study aimed to understand how microglial responses to hyperosmotic stress are regulated and whether they can be influenced by certain therapeutic agents, such as minocycline.
Exploring Hyperosmotic Stress and Microglia
Hyperosmotic stress refers to the imbalance of sodium concentrations outside cells, causing them to react and adjust. The researchers focused on NFAT5, a transcription factor known to regulate cellular responses to osmotic stress in various cell types but whose role in microglia was previously unclear. They aimed to determine if NFAT5 influences the expression of NOS2, an enzyme responsible for producing nitric oxide (NO), a key player in immune response and inflammation. The study also evaluated the effects of minocycline, an anti-inflammatory drug, on microglial activity under hyperosmotic stress conditions.
To investigate these mechanisms, the team subjected BV-2 microglial cells to increased sodium levels, 20 and 40 millimolar (mM) above normal concentrations. This allowed them to measure changes in NFAT5 expression, NOS2 production, and NO levels. They further explored how the Na+/Ca2+ exchanger (NCX) influenced this process and tested the effects of minocycline as a potential therapy.
The Role of NFAT5 in Microglial Response
The study revealed that both acute and chronic hyperosmotic stress significantly increased NFAT5 expression in BV-2 microglial cells. This rise in NFAT5 was associated with enhanced NOS2 expression and increased NO production, confirming that NFAT5 plays a pivotal role in modulating microglial responses under these conditions.
Interestingly, the study highlights that calcium ions (Ca2+) play a vital role in these reactions. When exposed to hyperosmotic stress, calcium efflux (release) through the Na+/Ca2+ exchanger, NCX, was observed. This efflux contributed to the upregulation of NOS2 and the production of NO. However, the researchers noted that this process might operate independently of NFAT5, suggesting that multiple pathways activate microglial responses duri
ng hyperosmotic stress.
Minocycline as a Potential Therapy
One of the most promising aspects of the study was the discovery of minocycline’s role in reducing the harmful effects of hyperosmotic stress on microglia. Minocycline significantly inhibited NOS2 expression and NO production in the stressed microglial cells. These findings suggest that minocycline could serve as a therapeutic agent for managing conditions related to high sodium levels in the brain.
However, the team found that minocycline’s inhibitory effects were independent of NFAT5, indicating the involvement of other underlying mechanisms. Despite this, minocycline’s effectiveness in dampening microglial activation presents a valuable therapeutic approach for hypernatremia-induced neurological disorders.
Acute vs. Chronic Hyperosmotic Stress: Differences in Microglial Behavior
The research team also delved into the differences between acute and chronic hyperosmotic stress, further expanding our understanding of microglial responses. Acute stress is typically associated with short-term exposure to high sodium levels, while chronic stress involves prolonged exposure over days or even weeks. The study found that both acute and chronic hyperosmotic stress led to increased NOS2 expression and NO production. However, chronic stress had a more significant effect on NO release compared to acute stress.
This finding is particularly important for understanding how long-term hypernatremia, often seen in patients with certain medical conditions, can lead to sustained neurological dysfunction. Understanding these mechanisms provides a clearer picture of how the brain adapts - or fails to adapt - to ongoing osmotic stress.
Expanding Therapeutic Research
While the study provided valuable insights into the role of NFAT5, NOS2, and NCX in microglial responses, the researchers acknowledged the need for further investigation into potential therapeutic agents. Minocycline’s effectiveness in inhibiting microglial activation presents a promising avenue for developing targeted therapies to address electrolyte imbalances and prevent their neurological consequences.
Future studies should explore how different therapeutic agents act on microglia under both acute and chronic hyperosmotic stress. This could lead to the development of more precise and effective treatments for patients suffering from hypernatremia-related conditions.
Conclusion: Toward Better Management of Hypernatremia
The study findings shed new light on the complex interplay between sodium concentrations and microglial activity, particularly through the modulation of NFAT5 and NCX. This understanding is crucial for researchers and clinicians tackling neurological dysfunctions associated with hypernatremia.
In summary, both acute and chronic exposure to elevated sodium levels caused increased NOS2 expression and NO production in microglia, mediated by NFAT5 and Ca2+ efflux through NCX. The findings also demonstrated the potential of minocycline as a therapeutic option for reducing these harmful effects, even though its mechanisms appear independent of NFAT5.
These discoveries underscore the importance of correcting hypernatremia to prevent potential neurological damage. As hypernatremia is associated with significant morbidity, particularly in its chronic form, these findings offer a pathway to better management and treatment of sodium imbalances in the brain.
The study findings were published in the peer-reviewed journal: Peptides.
https://www.sciencedirect.com/science/article/pii/S0196978124001207
For the latest on Brain Health, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/microglia-depletion-and-its-impact-on-glaucoma-progression
https://www.thailandmedical.news/news/herbs-and-phytochemicals-agathisflavone-from-catingueira-has-anti-inflammatory-effect-on-activated-microglia-in-neuroinflammation-and-alzheimer
