Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 03, 2024 5 months, 2 weeks, 1 day, 13 hours, 11 minutes ago
Medical News: Scientists from Nanjing Agricultural University and the Key Laboratory for Prevention and Control of Herbivorous Animal Diseases (Ministry of Agriculture and Rural Affairs) collaborated with the Xinjiang Animal Disease Research Key Laboratory to explore how Influenza A virus (IAV), specifically the WSN strain, manipulates the metabolic pathways of host cells to increase its replication rate. Their study, conducted on lab-grown human lung cells and infected mice, provides insight into how IAV triggers specific changes in host cell metabolism, helping the virus reproduce and spread more efficiently.
 How Influenza A Virus Manipulates Host Metabolism for Faster Replication
Influenza A Virus and Glycolysis
How Influenza A Virus Manipulates Host Metabolism for Faster Replication
Influenza A Virus and Glycolysis
The study focused on glycolysis, a primary metabolic process where cells break down glucose for energy. For the virus to replicate, it relies on the energy derived from the host cell's glucose metabolism. This
Medical News report sheds light on how influenza A virus hijacks glycolysis to ensure it has sufficient energy to multiply and thrive. Influenza A virus, known for causing seasonal flu in humans, can lead to a range of respiratory issues, and severe infections may result in complications, especially in vulnerable individuals.
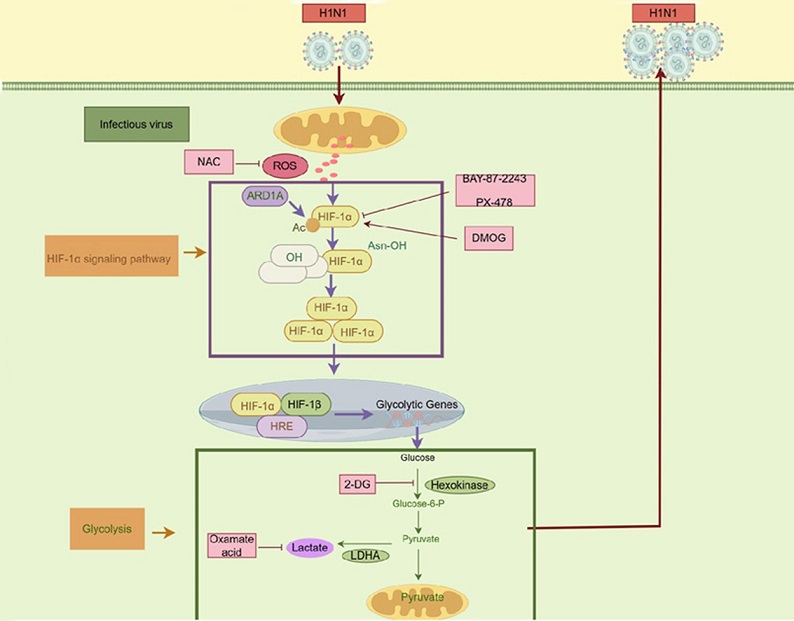
The researchers found that when the WSN virus infects lung cells (specifically A549 cells), it accelerates the glycolytic process, increasing the production of lactate - a byproduct of glycolysis. During the infection, two critical metabolic enzymes, hexokinase 2 (HK2) and lactate dehydrogenase A (LDHA), become highly active, driven by a specific pathway involving reactive oxygen species (ROS) and a protein called hypoxia-inducible factor-1 alpha (HIF-1α). This heightened activity essentially creates a cellular environment favorable for the virus to reproduce.
How ROS and HIF-1α Promote Virus Survival
One of the key discoveries in this research was the virus's activation of ROS and HIF-1α pathways to control glycolysis. ROS, or reactive oxygen species, are byproducts of cellular metabolism, especially in mitochondria, and while typically associated with cell damage, in this context, they seem to benefit the virus.
Increased ROS levels support the stability and activity of HIF-1α, a protein that regulates oxygen-dependent metabolic changes, including glycolysis.
As HIF-1α levels increase, it promotes the production of glycolytic enzymes, such as HK2 and LDHA, enabling a sustained environment for virus replication. The study demonstrated that when ROS levels were artificially decreased using a chemical called NAC (N-acetylcysteine), there was a corresponding decrease in HIF-1α levels. As a result, the glycolysis rate dropped, and the virus’s ability to replicate slowed down significantly.
In other words, Influenza A virus essentially induces a state within the host cell where glycolysis is boosted, allowing it to access more glucose-derived energy for its replication. This dependency on glucose metabolism suggests a potential target for treatments, as interrup
ting this process could reduce virus replication.
Testing Inhibitors on Infected Mice
To further explore possible treatments, the research team tested two types of inhibitors on mice infected with the WSN virus: a HIF-1α inhibitor called PTX-478 and a glycolysis inhibitor known as 2-Deoxyglucose (2-DG). Mice treated with these inhibitors showed marked improvements in symptoms. They experienced less weight loss - a common sign of severe infection - and exhibited less lung tissue damage compared to untreated mice.
This finding highlights a promising approach for managing Influenza A infections. By targeting HIF-1α and glycolysis pathways, it might be possible to limit the virus's ability to exploit host cell metabolism, thereby reducing the severity of infections.
Broader Implications for Antiviral Treatments
The study’s findings extend beyond the Influenza A virus, as similar metabolic manipulation strategies have been observed in other viruses. Some well-known viruses, such as Dengue virus (DENV), Marek’s disease virus (MDV), Newcastle disease virus (NDV), SARS-CoV-2, and African swine fever virus (ASFV), also rely on altering host cell metabolism to sustain their replication needs. In many cases, these viruses use glycolysis to produce energy or essential building blocks, which they then use for replication.
This study indicates that, like these viruses, Influenza A has developed sophisticated strategies to enhance glycolysis, allowing it to replicate more effectively. The reliance on glycolysis and ROS/HIF-1α signaling pathways might therefore represent a universal mechanism that viruses leverage to optimize their replication within host cells.
The Science Behind Virus Manipulation of Host Metabolism
According to the study, when a virus enters the body, it targets specific host cells and begins to take over their metabolic pathways. These infected cells must convert glucose into energy, primarily through glycolysis. Viruses use this metabolic conversion to gain energy while minimizing their reliance on oxygen, which would typically make it harder for the immune system to detect and combat the infection.
The research demonstrates that HIF-1α is a pivotal player in this process. As a transcription factor, HIF-1α activates genes responsible for increasing glycolytic enzymes under low-oxygen conditions. By using ROS to stabilize HIF-1α, Influenza A virus ensures a steady activation of glycolysis, producing lactate, which in turn promotes virus replication. The virus’s ability to manipulate these pathways reflects an adaptation strategy honed through evolution, making it highly adept at surviving and spreading.
Potential New Avenues for Influenza Treatment
The researchers propose that treatments designed to interfere with this pathway may hold promise for curbing virus replication and reducing symptoms in patients. By focusing on inhibitors that target HIF-1α or glycolysis, such as PTX-478 or 2-DG, scientists may be able to develop new therapies that could complement current antiviral drugs. This is especially important given that the influenza virus often mutates, potentially leading to strains that are resistant to existing antiviral medications.
Such treatments could benefit high-risk individuals, including those with weakened immune systems or chronic respiratory conditions, who are more susceptible to severe symptoms. By reducing virus replication within the host cells, the impact of the infection could be lessened, improving patient outcomes and alleviating healthcare burdens.
Conclusion
This study underscores the remarkable ways in which Influenza A virus manipulates the host cell environment to ensure its survival. By triggering glycolysis through ROS and HIF-1α pathways, the virus maximizes its replication potential, creating an internal system that favors its rapid multiplication. Future research will need to further investigate how inhibitors like PTX-478 and 2-DG can be applied in clinical settings, potentially leading to new treatment approaches that can effectively disrupt this viral survival mechanism.
In essence, these findings highlight an opportunity to explore metabolic pathways as therapeutic targets for influenza and other viral infections. If successful, this strategy could provide a new layer of protection against respiratory viruses, improving patient resilience and supporting better global health outcomes.
The study findings were published in the peer-reviewed Journal of Virology.
https://www.sciencedirect.com/science/article/abs/pii/S0891584924010128
For the latest on Influenza Research, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/antibiotics-during-flu-may-harm-lung-immunity-and-increase-bacterial-pneumonia-risk
https://www.thailandmedical.news/news/korean-researchers-find-that-the-herb-angelica-tenuissima-nakai-shows-promise-in-fighting-influenza-a
https://www.thailandmedical.news/articles/influenza-or-flu
