Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 17, 2024 1 year, 4 months, 3 weeks, 6 days, 8 hours, 37 minutes ago
Medical News: The study of how SARS-CoV-2 interacts with human cells has yielded significant insights into how this virus reprograms cellular metabolism to ensure its replication and survival. Researchers from the Technical University of Munich (TUM), the University of Würzburg, and Ludwig Maximilian University of Munich have come together to shed light on how SARS-CoV-2 exploits the metabolic pathways of human host cells. This
Medical News report explores the key findings of their research, focusing on how the virus manipulates energy production and resource allocation within the cells to support its own life cycle.
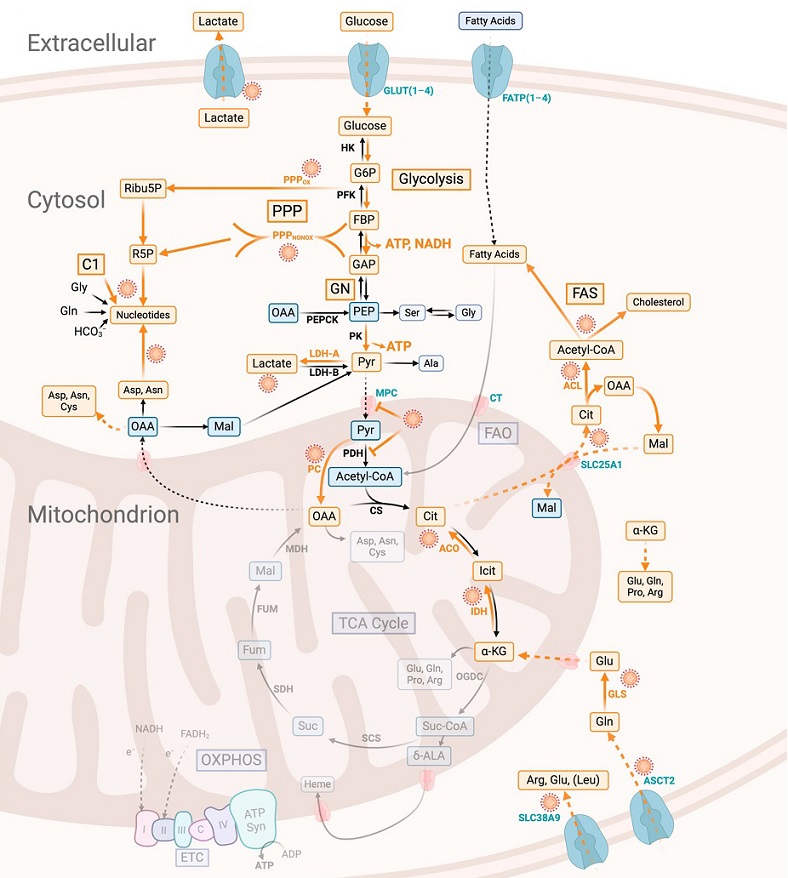 Metabolic program of SARS-CoV-2-infected cells suitable for viral replication. The major metabolic reactions supporting this program are marked by the virus symbol and broad orange arrows, and they include the following: (a) increased glucose uptake via the induction of glucose transporters, especially GLUT1 and GLUT3; (b) enhanced (aerobic) glycolysis due to increased PKM2, combined with increased lactate dehydrogenase A (LDH-A) activity, to regenerate NAD for the maintenance of glucose oxidation via glycolysis, which is necessary for the increased production of ATP, glucose-6-phosphate (G6P) (required for the initiation of PPP), and 3-phosphoglycerate (synthesis of serine/glycine, erythrose-4-phosphate); (c) activation of PPP with both arms to generate ribose-5-phosphate (R5P) and NADPH; (d) reduced OXPHOS via the inhibition of the pyruvate dehydrogenase (PDH)-mediated mitochondrial acetyl-CoA formation, which could be achieved either through the inhibition of the mitochondrial pyruvate carrier complex (MPC), which would block the entry of pyruvate (Pyr) into the mitochondria, or through the activation of pyruvate dehydrogenase kinase (PDHK), which would block the conversion of Pyr into acetyl-CoA; (e) specific metabolites normally produced in the TCA cycle and indispensable for SARS-CoV-2 replication, with α-KG, OAA, acetyl-CoA, citrate (Cit), and Suc-CoA especially able to be delivered via active TCA-cycle enzymes or various anaplerotic reactions, as α-KG can be provided via glutaminolysis, OAA via ATP-dependent PC and ATP-dependent Cit lyase (ACL), acetyl-CoA via fatty acid oxidation (FAO), Cit either via TCA-cycle enzymes from acetyl-CoA and OAA or reverse TCA-cycle reactions through the reduced carboxylation of α-KG and Suc-CoA via the TCA cycle enzyme α-KG dehydrogenase; (f) in the case of a glucose shortage, FAO is an alternative route for ATP generation.
The Virus and Its Target Cells
Metabolic program of SARS-CoV-2-infected cells suitable for viral replication. The major metabolic reactions supporting this program are marked by the virus symbol and broad orange arrows, and they include the following: (a) increased glucose uptake via the induction of glucose transporters, especially GLUT1 and GLUT3; (b) enhanced (aerobic) glycolysis due to increased PKM2, combined with increased lactate dehydrogenase A (LDH-A) activity, to regenerate NAD for the maintenance of glucose oxidation via glycolysis, which is necessary for the increased production of ATP, glucose-6-phosphate (G6P) (required for the initiation of PPP), and 3-phosphoglycerate (synthesis of serine/glycine, erythrose-4-phosphate); (c) activation of PPP with both arms to generate ribose-5-phosphate (R5P) and NADPH; (d) reduced OXPHOS via the inhibition of the pyruvate dehydrogenase (PDH)-mediated mitochondrial acetyl-CoA formation, which could be achieved either through the inhibition of the mitochondrial pyruvate carrier complex (MPC), which would block the entry of pyruvate (Pyr) into the mitochondria, or through the activation of pyruvate dehydrogenase kinase (PDHK), which would block the conversion of Pyr into acetyl-CoA; (e) specific metabolites normally produced in the TCA cycle and indispensable for SARS-CoV-2 replication, with α-KG, OAA, acetyl-CoA, citrate (Cit), and Suc-CoA especially able to be delivered via active TCA-cycle enzymes or various anaplerotic reactions, as α-KG can be provided via glutaminolysis, OAA via ATP-dependent PC and ATP-dependent Cit lyase (ACL), acetyl-CoA via fatty acid oxidation (FAO), Cit either via TCA-cycle enzymes from acetyl-CoA and OAA or reverse TCA-cycle reactions through the reduced carboxylation of α-KG and Suc-CoA via the TCA cycle enzyme α-KG dehydrogenase; (f) in the case of a glucose shortage, FAO is an alternative route for ATP generation.
The Virus and Its Target Cells
SARS-CoV-2, a positive-sense single-strand RNA virus, belongs to the same group as other pathogenic viruses such as hepatitis C, dengue, and Zika. When this virus infects human cells, it doesn’t simply take over the cell’s genetic machinery but also rewires the host's metabolic processes. The virus alters the central carbon metabolism, especially glycolysis, the pentose phosphate pathway (PPP), and the tricarboxylic acid (TCA) cycle, to maximize the production of key molecules like nucleotides, amino acids, and lipids. These molecules are crucial for the production of viral particles. The study exa
mines how the virus manipulates host-cell metabolism to ensure its replication and how these findings can potentially guide new therapeutic strategies.
Key Mechanisms: Hijacking Human Metabolism
One of the primary ways SARS-CoV-2 manipulates host cells is by forcing an increase in glucose uptake. Cells infected with the virus show heightened activity of glucose transporters like GLUT1 and GLUT3, leading to an increase in glycolysis. This process provides the virus with a rapid supply of ATP, the energy currency of cells, which is essential for synthesizing the macromolecules needed for viral replication. Aerobic glycolysis, as induced by the virus, also leads to increased production of lactate, which helps regenerate NAD+ for continued glycolysis.
SARS-CoV-2 also taps into the host's amino acid supply. Infected cells often show elevated levels of amino acids, which are necessary for viral protein production. The virus further enhances nucleotide synthesis by activating the pentose phosphate pathway (PPP), which is key to producing the ribose-5-phosphate required for nucleotide biosynthesis.
Another notable metabolic alteration is the virus’s manipulation of fatty acid and lipid metabolism. Since the virus forms new particles that need to be enveloped in lipid membranes, it increases the host's fatty acid synthesis pathways, ensuring a steady supply of lipids.
Virus-Induced Suppression of Host Defenses
SARS-CoV-2 does not stop at hijacking the metabolic machinery; it also suppresses the host's defense systems. One key mechanism involves the inhibition of autophagy, a cellular process that typically helps cells break down and recycle damaged components, including viral particles. By suppressing autophagy, the virus ensures that newly formed viral particles remain intact and can continue to replicate.
The virus also interferes with the host's antiviral response by inhibiting p53, a protein that plays a central role in regulating cell death and the immune response to viral infections. Studies show that when p53 is suppressed, as it is in SARS-CoV-2 infections, viral replication increases. The viral proteins NSP1, NSP5, and ORF7a are directly involved in this suppression, effectively limiting the cell's ability to launch a robust immune response.
Viral Protein Interactions with Human Cells
The spike (S) protein of SARS-CoV-2 plays a crucial role in viral entry into host cells. This protein binds to the ACE2 receptor on human cells, allowing the virus to fuse with the cell membrane and release its RNA into the cell's cytoplasm. But the S protein doesn’t stop there; it also interacts with other receptors such as the glucose-regulated protein 78 (GRP78) and C-type lectin receptors. These interactions facilitate the virus's entry into various cell types, even in cells that may not express ACE2.
Once inside the host cell, the viral RNA is translated into proteins that drive the replication of the viral genome. Proteins like NSP12 (the RNA-dependent RNA polymerase), NSP7, and NSP8 form a complex that synthesizes new viral RNA, ensuring that the virus can continue to replicate. To meet the high energy demands of these processes, the virus manipulates cellular metabolism, rerouting resources away from normal cellular functions and toward viral replication.
Therapeutic Implications
Understanding how SARS-CoV-2 reprograms host-cell metabolism has opened new avenues for therapeutic interventions. By targeting key metabolic pathways, researchers hope to develop treatments that can disrupt the virus’s life cycle without harming the host. For example, studies have shown that inhibiting glycolysis with the drug 2-deoxyglucose (2-DG) drastically reduces viral replication. Similarly, targeting the pentose phosphate pathway or fatty acid synthesis could help starve the virus of the resources it needs to reproduce.
Conclusion
The research from these leading institutions highlights the importance of metabolic reprogramming in SARS-CoV-2 infections. By altering the host’s glucose, amino acid, and lipid metabolism, the virus ensures it has the necessary building blocks for replication. At the same time, it suppresses the host’s immune response, allowing for unchecked viral growth. This understanding of the virus’s interaction with host metabolism offers promising targets for new treatments aimed at disrupting these processes. By focusing on metabolic pathways, future therapies could not only reduce viral replication but also mitigate the severe outcomes of COVID-19.
The study findings were published in the peer-reviewed International Journal of Molecular Sciences.
https://www.mdpi.com/1422-0067/25/18/9977
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-india-approves-2-deoxy-d-glucose-2-dg-as-an-adjuvant-to-treat-covid-19-patients-and-reduce-supplemental-oxygen-dependence
https://www.thailandmedical.news/news/philadelphia-study-validates-that-sars-cov-2-causes-mitochondrial-metabolic-and-epigenomic-reprogramming
