Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 18, 2024 3 months, 3 weeks, 5 days, 41 minutes ago
Medical News: The COVID-19 pandemic, caused by the SARS-CoV-2 virus, took the world by storm at the end of 2019, infecting millions and causing widespread disruptions to health systems globally. With over 780 million confirmed cases and more than 8 million deaths reported worldwide, the virus's rapid spread has been attributed not only to its infectious nature but also to its unique ability to outwit the human immune system. Researchers from Renmin Hospital of Wuhan University and the State Key Laboratory of Virology at Wuhan University in China have been investigating how SARS-CoV-2 manages to evade innate immunity - the body's first line of defense. This
Medical News report dives deep into the virus’s strategies to bypass immune responses and explores the implications of its evolution.
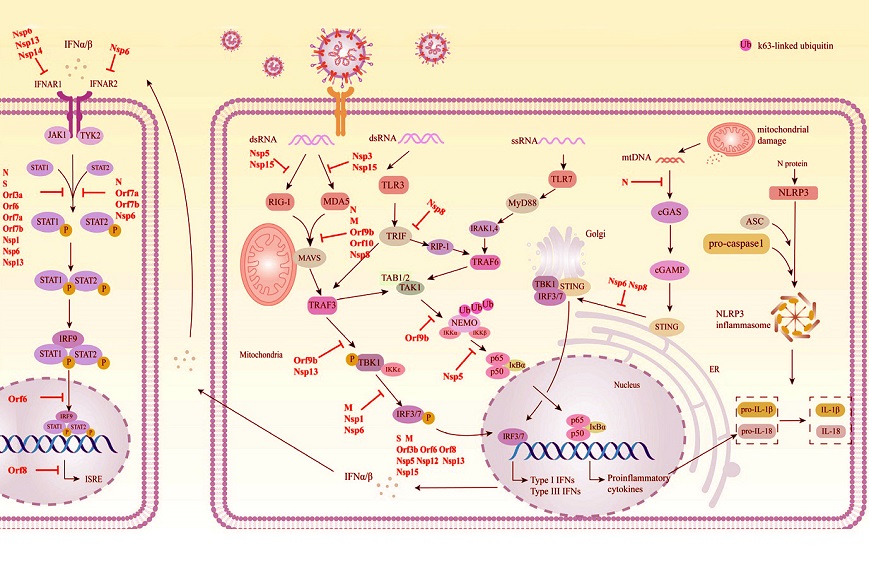 Schematic diagram of the mechanism by which SARS-CoV-2 evades innate immunity. SARS-CoV-2 proteins that target individual pathways are highlighted in red.
Understanding the Basics of Immunity and SARS-CoV-2 Infection
Schematic diagram of the mechanism by which SARS-CoV-2 evades innate immunity. SARS-CoV-2 proteins that target individual pathways are highlighted in red.
Understanding the Basics of Immunity and SARS-CoV-2 Infection
The human immune system recognizes and fights viral invaders like SARS-CoV-2 using a process called the innate immune response. When the virus enters human cells through the ACE2 receptor, a series of signals are triggered, alerting immune cells to the presence of a pathogen.
These signals activate pattern recognition receptors (PRRs), which identify the virus's genetic material as foreign. PRRs then activate the production of interferons (IFNs), essential proteins that alert surrounding cells and induce the creation of hundreds of antiviral molecules.
This study review reveals that SARS-CoV-2 has cleverly evolved multiple mechanisms to disable key components of this innate immune response. Its 29 viral proteins are highly specialized and contribute not only to viral replication but also to suppressing immune signaling pathways. This ability allows the virus to persist in the host for longer periods, increasing its spread and severity.
The Role of Viral Proteins in Suppressing Immune Responses
Researchers have identified several SARS-CoV-2 proteins - both structural and non-structural - that interfere with immune system functions. These proteins block various steps in the interferon signaling pathways and prevent antiviral defenses from being activated effectively. Here are some key findings:
-Nsp15 and Evasion of PRR Recognition
The non-structural protein Nsp15 helps the virus remain undetected by PRRs. During viral replication, it acts like a molecular scissor, cleaving the virus’s RNA into smaller pieces to prevent the immune system from identifying it as a threat. This action reduces the immune system’s ability to detect viral material and mount an effective response.
-Blocking Type I Interferon Production
Several SARS-CoV-2 proteins target the production of interferons. For example:
N protein and Nsp5 hinder RIG-I, a key sensor for detecting viral RNA, preventing t
he activation of signaling pathways.
Orf9b disrupts the interaction between TOM70 and HSP90, two proteins needed to activate immune signals.
The M protein and Orf8 interfere with critical steps, stopping interferon production.
-Shutting Down JAK-STAT Signaling
The JAK-STAT pathway is responsible for amplifying interferon signals, which prompt nearby cells to defend themselves. SARS-CoV-2 proteins, such as the N protein, S protein, Orf7a, and Nsp13, block this pathway at multiple points. By halting JAK-STAT activity, the virus reduces the production of protective proteins, making the body more vulnerable.
-Preventing Nuclear Import and Export
To fight infections, immune signaling molecules like STAT1 and IRF3 must move into the cell nucleus. The Orf6 protein targets and blocks the nuclear pore complex, preventing these molecules from entering the nucleus. Other proteins, including Nsp1 and Nsp9, further interfere with nuclear export systems, stopping the production of antiviral proteins altogether.
-Autophagy Suppression
Autophagy is the body’s mechanism for clearing out damaged cells and pathogens. SARS-CoV-2 uses the Orf3a protein to disrupt this process by blocking essential cellular components needed for autophagy to occur. The virus also uses Nsp6 to degrade molecules like STING, which play a role in activating immune defenses.
How Omicron Variants Changed the Game
As SARS-CoV-2 spread globally, it continued to mutate, leading to the emergence of new variants. Among these, the Omicron variant marked a major shift in the virus’s evolution. Omicron has over 30 mutations in its spike protein, which helps it evade antibodies from vaccines or prior infections. However, its ability to escape immune defenses goes beyond spike mutations.
Recent studies showed that Omicron variants such as BA.4, BA.5, and XBB.1.5 have higher levels of Orf6 and N protein expression, enhancing their ability to block interferon responses. This increased expression allows the virus to evade detection more effectively and maintain higher levels of infection.
Interestingly, mutations in other proteins like Nsp6 have reduced their immune-evasion abilities. For instance, the triple amino acid deletion in Omicron’s Nsp6 protein makes it less effective at degrading STING. Researchers speculate that this might explain why Omicron infections often cause milder symptoms compared to earlier strains, even though it spreads more quickly.
The Bigger Picture and Implications
Understanding how SARS-CoV-2 evolves to evade the immune system highlights critical challenges for developing treatments and vaccines. The virus’s ability to block interferon production, shut down immune pathways, and suppress cellular defenses gives it a significant advantage. Researchers believe that targeting these immune evasion mechanisms - for example, by inhibiting viral proteins like Orf6, Nsp15, or Nsp5 - could lead to new therapeutic strategies.
The study also underscores the importance of monitoring viral evolution beyond the spike protein. While most attention has focused on spike mutations, non-spike proteins like Nsp6 and Orf6 play equally vital roles in immune evasion and adaptation.
Conclusions
The SARS-CoV-2 virus continues to demonstrate remarkable adaptability in evading the body’s innate immune system. Through its structural and non-structural proteins, the virus disrupts interferon production, signaling pathways, and cellular defenses at multiple levels. These strategies ensure viral survival, replication, and spread while reducing the host’s ability to mount a robust immune response.
The Omicron variant represents a key milestone in the virus’s evolution. By enhancing the expression of proteins like Orf6 and N, Omicron has improved its ability to evade innate immunity. However, mutations in other proteins, such as Nsp6, may reduce the virus’s capacity to suppress immune responses, offering some hope for less severe disease outcomes in future variants. Understanding these immune evasion mechanisms will be essential for the development of better antiviral therapies and vaccines.
The study findings were published in the peer-reviewed journal: Pathogens.
https://www.mdpi.com/2076-0817/13/12/1117
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-xec-variant-shows-enhanced-immune-evasion-through-ntd-glycosylation
https://www.thailandmedical.news/news/sars-cov-2-uses-heme-and-complement-factor-h-for-immune-evasion
https://www.thailandmedical.news/articles/coronavirus
