Nikhil Prasad Fact checked by:Thailand Medical News Team May 20, 2024 10 months, 4 weeks, 2 days, 4 hours, 55 minutes ago
COVID-19 News: SARS-CoV-2, the virus responsible for COVID-19, is primarily known for attacking the lungs. However, it doesn’t stop there. This virus also targets other organs, including the kidneys, gastrointestinal tract, heart, skin, and notably, the brain. But how exactly does SARS-CoV-2 infiltrate the brain? This question has puzzled scientists and clinicians alike. Recent research from the University of South Florida and the James A Haley VA Hospital in Tampa that is covered in this
COVID-19 News report, aims to shed light on this mystery by exploring how the virus crosses the blood-brain barrier (BBB).
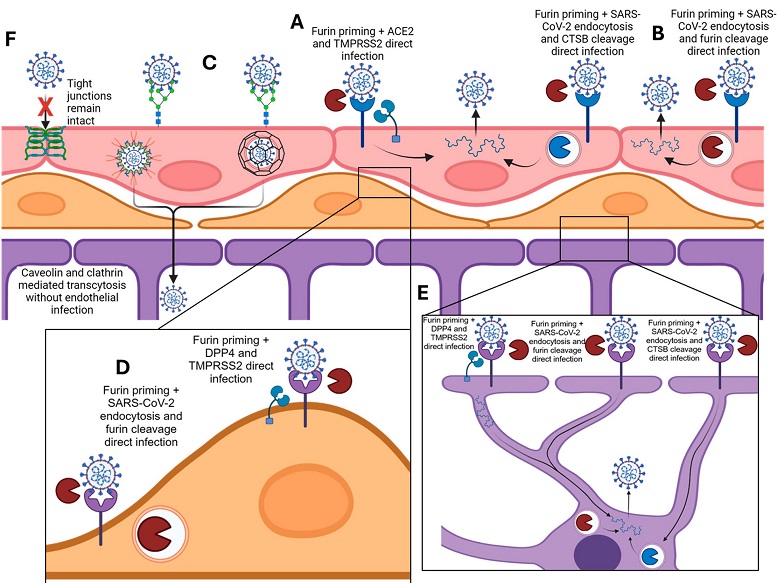 Proposed model of SARS-CoV-2 infection and transcytosis of the BBB. (A,B) Direct infection of brain endothelial cells including ACE2 binding, furin priming, and S1/S2 cleavage by either TMPRSS2, or CTSB and/or furin. (C) Transcytosis with initial anchoring to glycocalyx, endocytosis by either caveolin or clathrin, and deposition on abluminal side of endothelium. (D,E) Proposed mode of infection of pericytes and astrocytes utilizing DPP4 binding, furin priming, and TMPRSS2 spike cleavage, or DPP4 binding and endocytosis for spike cleavage within endosome. (F) Diagram showing intact tight junctions preventing passive diffusion of SARS-CoV-2.
The Blood-Brain Barrier: A Guardian of the Brain
Proposed model of SARS-CoV-2 infection and transcytosis of the BBB. (A,B) Direct infection of brain endothelial cells including ACE2 binding, furin priming, and S1/S2 cleavage by either TMPRSS2, or CTSB and/or furin. (C) Transcytosis with initial anchoring to glycocalyx, endocytosis by either caveolin or clathrin, and deposition on abluminal side of endothelium. (D,E) Proposed mode of infection of pericytes and astrocytes utilizing DPP4 binding, furin priming, and TMPRSS2 spike cleavage, or DPP4 binding and endocytosis for spike cleavage within endosome. (F) Diagram showing intact tight junctions preventing passive diffusion of SARS-CoV-2.
The Blood-Brain Barrier: A Guardian of the Brain
The BBB is a complex system that protects the brain from harmful substances in the bloodstream while allowing essential nutrients to pass through. It consists of three main cell types: endothelial cells that line the blood vessels, pericytes that wrap around these vessels, and astrocytes that provide support. This barrier is crucial for maintaining the brain’s delicate environment.
The Study: Investigating SARS-CoV-2’s Entry Points
The study focused on how SARS-CoV-2 interacts with the BBB and its components: human umbilical vein endothelial cells (HUVECs), astrocytes, and pericytes. Researchers specifically looked at the roles of two receptors, ACE2 and DPP4, which the virus might use to enter these cells.
Key Findings
-Infection Susceptibility: All three cell types - HUVECs, astrocytes, and pericytes - are susceptible to SARS-CoV-2 infection. This was determined by exposing these cells to the virus and observing infection markers.
-Role of ACE2 and DPP4: HUVECs express the ACE2 receptor, which is already known to be a primary entry point for the virus in lung cells. Astrocytes and pericytes, however, do not express ACE2 but do express DPP4, another potential entry receptor.
-Endocytosis Pathways: The virus uses clathrin and caveolin-mediated endocytosis - processes by which cells internalize molecules—to cross the BBB without necessarily disrupting its tight junctions.
Detailed Analysis: Br
eaking Down the Processes
To determine if the BBB cells could be infected by SARS-CoV-2, researchers used a transwell model - a system that mimics the BBB. They exposed monolayers of HUVECs, astrocytes, and pericytes to the virus and monitored the infection. Fluorescent imaging revealed that the virus successfully infected these cells, albeit with varying levels of efficiency.
Impact of ACE2 and DPP4
By using lentiviruses to knock down ACE2 in HUVECs and DPP4 in astrocytes and pericytes, researchers found that reducing these receptors decreased, but did not completely prevent, infection. This suggests that while ACE2 and DPP4 play significant roles in the virus's entry, other factors are also involved.
Transcytosis Across the BBB
One of the most intriguing findings was that SARS-CoV-2 can traverse the BBB via transcytosis—an active transport mechanism - rather than by disrupting the barrier’s tight junctions. This process involves the virus being engulfed by cells and transported across them in vesicles.
Role of Endocytosis
To pinpoint how the virus crosses the BBB, researchers used inhibitors of endocytosis and endosomal transport. They discovered that clathrin and caveolin - proteins involved in forming vesicles for transport within cells—are crucial for the virus’s movement across the BBB.
Implications and Future Directions
These findings have significant implications for understanding how SARS-CoV-2 infects the brain and potentially causes neurological symptoms associated with COVID-19. The research highlights the complex interactions between the virus and the BBB, suggesting that targeting these pathways could help develop treatments to protect the brain from infection.
Potential Treatments
Future research could explore ways to block the virus’s entry into BBB cells by inhibiting clathrin and caveolin-mediated endocytosis. Additionally, understanding the role of DPP4 in astrocytes and pericytes might lead to new therapeutic targets.
Broader Impact
This study also underscores the importance of considering the BBB's unique environment when developing models to study brain infections. Many existing models lack the complexity of the BBB’s tri-cellular structure, which is critical for accurate representation of in vivo conditions.
Conclusion
SARS-CoV-2’s ability to cross the BBB and infect the brain is a multifaceted process involving various cellular mechanisms. This research provides crucial insights into how the virus interacts with the BBB and highlights potential avenues for therapeutic intervention. As we continue to grapple with the long-term effects of COVID-19, understanding these mechanisms will be essential for mitigating the impact of the virus on the brain.
The study findings were published in the peer reviewed journal: Viruses.
https://www.mdpi.com/1999-4915/16/5/785
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
