How Skin Color Can Influence Drug Efficacy - New Insights into Medication Response
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 14, 2024 1 year, 2 months, 10 hours ago
Medical Research News: How Skin Pigmentation Affects Medication
A groundbreaking new study is shedding light on how skin pigmentation may play a surprising role in how well certain medications work. According to researchers from the University of California, Riverside, and others, melanin, the pigment that gives skin its color, could act as a "sponge" for some drugs, influencing how quickly these medications reach their intended targets in the body. This
Medical Research News report aims to provide an easy-to-understand overview of the study's findings, which could have a significant impact on how drugs are prescribed based on a person's skin tone.
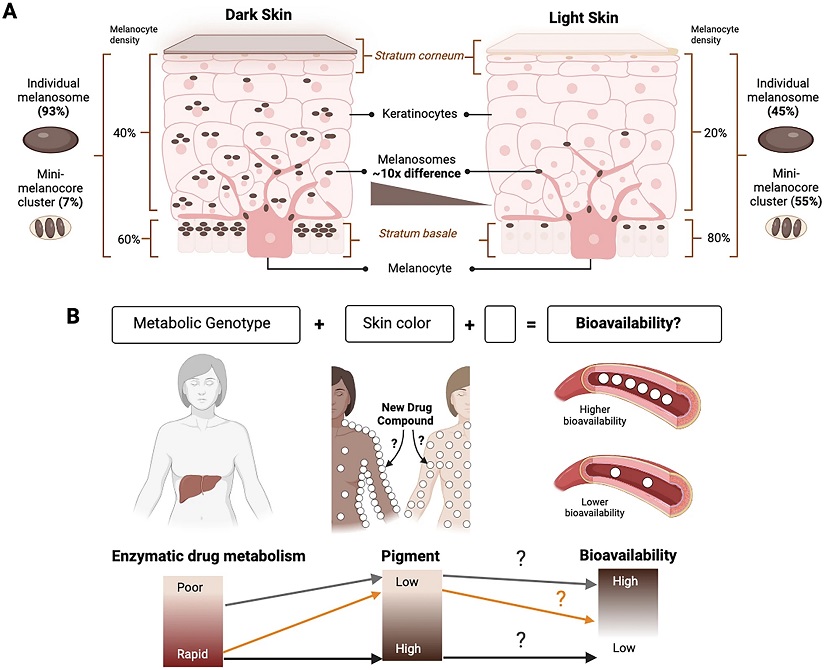 A The skin is composed of multiple layers, from the innermost stratum basale that contains the melanocytes to intermediate layers dominated by keratinocytes and the outermost stratum corneum. The melanocytes produce the melanin-containing melanosomes, which show an approximately 10 times difference in accumulation between individuals with dark and light skin tones. The melanosomes are transferred to the keratinocytes, where they surround the nucleus and protect DNA from UV-induced damage. B This simplified model posits that differential drug responses between human genetic ancestry groups [1] could result from influences of a combination of both the genotype for drug metabolic enzymes and skin eumelanin level of an individual (visible as skin tone) on the bioavailability of drugs. The empty block in the model represents known and/or unknown other variables that may further influence drug bioavailability
A The skin is composed of multiple layers, from the innermost stratum basale that contains the melanocytes to intermediate layers dominated by keratinocytes and the outermost stratum corneum. The melanocytes produce the melanin-containing melanosomes, which show an approximately 10 times difference in accumulation between individuals with dark and light skin tones. The melanosomes are transferred to the keratinocytes, where they surround the nucleus and protect DNA from UV-induced damage. B This simplified model posits that differential drug responses between human genetic ancestry groups [1] could result from influences of a combination of both the genotype for drug metabolic enzymes and skin eumelanin level of an individual (visible as skin tone) on the bioavailability of drugs. The empty block in the model represents known and/or unknown other variables that may further influence drug bioavailability
The researchers behind this study argue that many drugs can bind to melanin in the skin, which could lead to differences in how effective these medications are in people with varying skin tones. “Melanin, responsible for skin color, has a surprising affinity for certain drugs,” explained Simon Groen, assistant professor of evolutionary systems biology at the University of California, Riverside. This study explores how this phenomenon might affect drug safety and dosing, as skin tone plays a role that has been largely overlooked.
Melanin and Medication: The Science Behind the Study
According to Groen and his co-author Sophie Zaaijer, who specializes in diversity and inclusion in clinical trials, current drug development practices often fail to account for the effects of skin pigmentation on drug interactions. This oversight is especially concerning as clinical trials become more diverse, including participants of different racial and ethnic backgrounds. While the U.S. Food and Drug Administration (FDA) has guidelines to ensure safety in drug testing, these guidelines do not specifically address how skin pigmentation might affect drug interactions.
One of the examples highlighted in the study involves nicotine. The researchers found that nicotine, commonly consumed through smoking or nicotine patches, can bind to skin pigments. This binding may alter how effective nicotine patches are in helping people with darker skin tones quit smoking. If the patches don’t work as effectively for individuals with more melanin, it could mean that smokers with darker skin are at a dis
advantage when it comes to using this form of smoking cessation aid.
A New Approach to Drug Testing
The researchers propose a new workflow that pharmaceutical companies could adopt to address this issue. This would involve using 3D skin models with varying pigmentation levels to test how different drugs bind to melanin. By doing this, drug developers could better understand how skin color affects drug efficacy and make more informed decisions when determining safe and effective dosages for people of all skin tones.
“Skin pigmentation should be considered a factor in both safety and dosing estimates,” Zaaijer said. She believes that the biomedical industry is on the cusp of a transformative era where inclusivity will become a necessity, not just an option. The researchers argue that the push for more diversity in clinical trials will require a complete overhaul of how drugs are tested and dosed across different populations.
Skin Tone, Genetics, and Drug Response
The role of skin pigmentation in drug interactions is just one example of how genetic differences among racial and ethnic groups can lead to vastly different drug responses. The study suggests that up to 20% of all medications may be affected by these genetic variations. However, our understanding of these differences remains limited.
Zaaijer noted that to address this issue, it’s essential to make changes not just in drug development but in the regulatory process as well. She emphasized the importance of collaboration between academic researchers, pharmaceutical companies, clinicians, and regulatory agencies like the U.S. FDA.
Legislative Changes and Future Directions
A new law, the Food and Drug Omnibus Reform Act, which was enacted in 2022, has already set the stage for more inclusive drug development practices. This law mandates that clinical trials must consider patient diversity. In addition, the FDA has recently published draft guidelines that are expected to be finalized soon. These guidelines will require drug developers to consider various pharmacokinetic variables, such as how drugs move through the body in patients of different ancestries.
Once these guidelines are finalized, they will help ensure that drugs are tested more equitably across diverse populations. The researchers hope this will lead to more systematic evaluations of how skin pigmentation and genetic factors affect drug response.
Practical Implications for Patients and Clinicians
The study also highlights the importance of making patients and healthcare providers aware of the potential differences in drug efficacy based on skin tone. Patients, particularly those from minority backgrounds, should feel empowered to ask their doctors whether a drug has been tested for safety and efficacy in people with similar genetic and skin tone backgrounds.
Groen pointed out that certain genetic variations are more common in specific ancestral groups, which can influence how a drug is metabolized. When these differences are considered early in the drug development process, patients are likely to trust the process more and be more willing to participate in clinical trials. The researchers believe that fostering this trust is crucial for the future of medicine.
The Importance of Skin Models in Drug Development
The researchers emphasize that implementing 3D skin models with different pigmentation levels could be a game-changer in preclinical research. These models can mimic the way drugs interact with different skin types and provide valuable insights into potential safety concerns or required dosage adjustments for people with varying melanin levels.
By incorporating skin pigmentation as a variable in drug testing, pharmaceutical companies could create more inclusive drugs that work effectively across all population groups, regardless of their skin tone or genetic background.
Conclusion: A Step Towards More Inclusive Medicine
The findings of this study bring to light the importance of considering skin pigmentation in drug development and testing. As the world moves toward more diverse clinical trials, it is vital to understand how skin tone and genetic differences can impact medication response. This study not only highlights the gaps in current FDA guidelines but also offers a practical solution for addressing these issues through the use of 3D skin models and more inclusive drug testing practices.
In the future, the success of medical treatments may depend on our ability to create drugs that are effective for people of all skin tones. This will require a shift in how we approach drug development, testing, and clinical trials. By embracing inclusivity, we can ensure that medications are safe and effective for everyone, regardless of their skin color or genetic background.
The study findings were published in the peer-reviewed journal: Human Genomics.
https://humgenomics.biomedcentral.com/articles/10.1186/s40246-024-00677-7
For the latest
Medical Research News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-inhibits-cytochrome-p450-enzymes-that-metabolize-drugs-like-antipsychotics-leading-to-higher-blood-levels
https://www.thailandmedical.news/news/breaking-a-must-for-all-doctors-to-read-scientists-warn-that-covid-19-affects-cytochrome-p450-3a4-mediated-drug-metabolism-and-drug-interactions
