Immunology News: Regulation Of Cytotoxic T-Cell Differentiation And Exhaustion By mTOR Signaling -Unraveling the Complexities Of Adaptive Immunity
Thailand Medical News Team Aug 19, 2023 2 years, 4 months, 1 week, 20 hours, 56 minutes ago
Immunology News: Cytotoxic T lymphocytes (CTLs) stand as the vanguard of adaptive immunity, poised to combat tumors and viral invaders. Emerging from the thymus gland, these cells respond swiftly upon encountering antigens, orchestrating a defense that safeguards the host. A nuanced understanding of the molecular mechanisms underlying both exhausted and memory CTLs is pivotal in crafting interventions rooted in cell-mediated immunity.
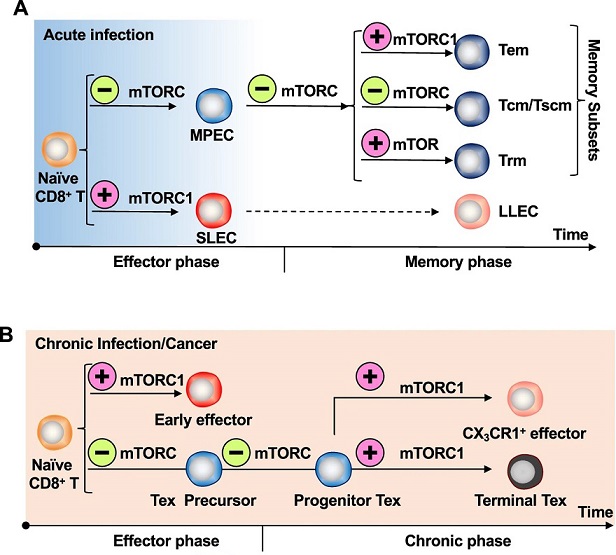 A) During acute infection, virus-specific naïve CD8+ T cells differentiate into SLECs that display potent cytotoxicity and MPECs that have a greater capacity to form memory cells following viral clearance. After the antigen has been eliminated, a minority of SLECs manage to survive and transform into LLECs. On the other hand, MPECs develop into various types of memory T cells, including Tem, Tcm, and Trm. mTORC1 activity instructs SLEC and Tem differentiation. Inhibition of mTOR, either by Rapamycin treatment or through siRNA-mediated knockdown, promotes MPEC and memory formation, particularly for Tcm and human Tscm. However, mTOR activation in T cells promotes their differentiation into Trm cells and enhances their survival in peripheral tissues. B) During chronic infection or cancer, virus-specific naïve CD8+ T cells are activated and segregate into early effector cells and precursor cells. The precursor cells develop into Progenitor Tex, which further differentiate into terminally exhausted cells and effector-like cells marked by CX3CR1 expression. Inhibition of mTOR activity enhances Progenitor Tex formation at both the early and late stages.
A) During acute infection, virus-specific naïve CD8+ T cells differentiate into SLECs that display potent cytotoxicity and MPECs that have a greater capacity to form memory cells following viral clearance. After the antigen has been eliminated, a minority of SLECs manage to survive and transform into LLECs. On the other hand, MPECs develop into various types of memory T cells, including Tem, Tcm, and Trm. mTORC1 activity instructs SLEC and Tem differentiation. Inhibition of mTOR, either by Rapamycin treatment or through siRNA-mediated knockdown, promotes MPEC and memory formation, particularly for Tcm and human Tscm. However, mTOR activation in T cells promotes their differentiation into Trm cells and enhances their survival in peripheral tissues. B) During chronic infection or cancer, virus-specific naïve CD8+ T cells are activated and segregate into early effector cells and precursor cells. The precursor cells develop into Progenitor Tex, which further differentiate into terminally exhausted cells and effector-like cells marked by CX3CR1 expression. Inhibition of mTOR activity enhances Progenitor Tex formation at both the early and late stages.
A recent review study conducted by researchers from Shanghai Jiao Tong University School of Medicine-China, delves into the intricate interplay of molecular signaling governed by the mammalian target of rapamycin (mTOR), with a particular emphasis on mTOR complex 2 (mTORC2), within the realm of memory and exhausted CD8+ T lymphocytes.
Cytotoxic T-Cell Differentiation and the Crossroads of Exhaustion
The journey of CTLs is characterized by differentiation into effector cells during acute infections, and memory cells, offering sustained immune protection. However, chronic infections and malignancies pave the way for CTLs to progressively transition into a dysfunctional exhausted state. This complex landscape is underscored by the diversity within pools of exhausted and memory CTLs.
As covered in previous studies and
Immunology News reports, T-cell exhaustion, a state borne from chronic antigen stimulation and inflammation, manifests as a loss of effector function, accompanied by the upregulation of inhibitory receptors. T-cell receptor (TCR) stimulation plays a pivotal role in exhaustion, giving rise to a unique cytotoxic subset known as exhausted (Tex) cells. These Tex cells exhibit diminished cytokine production and a reduction in cytotoxic effector molecules.
Within the realm of exhausted CTLs, a heterogeneity of subsets emerges. The journey of CTLs from chronic infections or tumor growth culminates in the formation of progenitor-exhausted T lymphocytes (progenitor Tex cells), characterized by high Ly108 expression and low CX3C m
otif chemokine receptor 1 (CX3CR1) and killer cell lectin-like receptor G (KLRG) expression. The initiation of TCR signaling sets the stage for downstream transcription factor activation, propelling the differentiation of progenitor Tex cells into full-fledged Tex cells.
Metabolic Crossroads: mTOR's Role in Cytotoxic T-Cell Differentiation
Metabolism lies at the heart of T-cell activity, shifting from oxidative phosphorylation (OXPHOS) in quiescent naive CTLs to a dynamic interplay between aerobic glycolysis and OXPHOS upon activation. Effector cells predominantly adopt aerobic glycolysis, while memory CTLs favor enhanced OXPHOS and fatty acid oxidation. However, in the realm of chronic infections and tumorigenesis, these metabolic pathways are disrupted, leading to a heightened dependence on glycolysis for Tex cells.
At the nexus of these metabolic intricacies stands mTOR, orchestrating the differentiation of CTLs. The mTOR complexes, mTORC1 and mTORC2, wield differential control over CTL fate and memory T-cell formation. While mTORC1 steers cell development and metabolism, mTORC2 governs cell viability and cytoskeletal architecture. An intricate dance of reciprocal regulation between the two complexes unfolds through S6K phosphorylation and TSC complex inhibition.
The Symphony of Differentiation: mTOR's Orchestration of CTL Fate
During acute infections and vaccinations, the differentiation of effector CD8+ T lymphocytes begets two distinct subsets: short-lived effector cells (SLECs) and memory precursor effector cells (MPECs). These subsets, equipped with akin cytotoxic effector functions, navigate the aftermath of the effector phase. Most SLECs meet their demise through apoptosis, with a minority persevering to evolve into long-lived effector cells (LLEs).
The odyssey of MPECs unfolds as they give rise to central memory T (Tcm), resident memory T (Trm), and effector memory T (Tem) lymphocytes. An intriguing player, the Sin1/mTORC2 complex, wields influence over the GATOR1-KICSTOR complex, regulating cell growth, metabolism, immune responses, and tumorigenesis. The administration of rapamycin nurtures the MPEC phenotype, fortifying memory precursor T cells, and magnifying the efficacy of anti-programmed cell death ligand 1 (PD-L1) therapy by augmenting proliferation.
Unraveling the Intricacies: Charting Future Avenues
The terrain of immunological memory and exhaustion remains a tantalizing realm for exploration. The panorama of CD8+ T-cell subsets, in all their heterogeneity, has been illuminated, yet the elucidation of the molecules and pathways steering their fate persists as a paramount endeavor. At the helm of this orchestration stands mTOR signaling, a linchpin integrating multifarious pathways and engendering a pivotal role in T-cell function.
The intricacies governing precise mTOR activity regulation in CD8+ T-cell subsets beckon further inquiry. Discerning the specific roles of mTORC1 and mTORC2 in the annals of memory T-cell and Tex cell development remains a realm ripe for exploration, especially unraveling the nuances of mTORC2- and Sin1-mediated mTORC2 signaling's orchestration of T-cell exhaustion.
Recent revelations spotlight the epigenetic sway of the mTOR pathway in sculpting T cells, exerting dominion over enzymes that mold chromatin structure and DNA methylation, thereby sculpting gene expression. This realm beckons for deeper exploration to fathom its ramifications on T-cell function across health and ailment.
In the realm of metabolism, mTOR's status as a conductor of metabolic processes remains undiminished. By sensing shifts in metabolite levels, particularly glucose and amino acids, mTOR wields dominion over an array of metabolic enzymes. The riddle of how mTOR signaling precisely molds glycolytic pathways, oxidation, and mitochondrial function within T cells still beckons for an unveiling.
In sum, the story of mTOR's role in the panorama of cytotoxic T-cell differentiation and exhaustion stands as a pivotal chapter in the tale of adaptive immunity. As we probe deeper, we inch closer to a future where the orchestration of cellular metabolism holds the key to rekindling the flames of exhausted T-cell function, safeguarding the body against the relentless onslaught of pathogens and maladies.
The study review was published in the peer reviewed journal: Cellular & Molecular Immunology.
https://www.nature.com/articles/s41423-023-01064-3
For the latest
Immunology News, keep on logging to Thailand Medical News.
