Induced Macropinocytosis, A Rare Form Of Cell Death May Help In New Cancer Therapy Protocols
Source: Thailand Medical News Dec 20, 2019 5 years, 4 months, 1 week, 3 hours, 23 minutes ago
New research show that in laboratory experiments, a metabolic inhibitor was able to kill a variety of human
cancer cells of the skin, breast, lung, cervix and soft tissues through a non-apoptotic route called catastrophic
macropinocytosis.
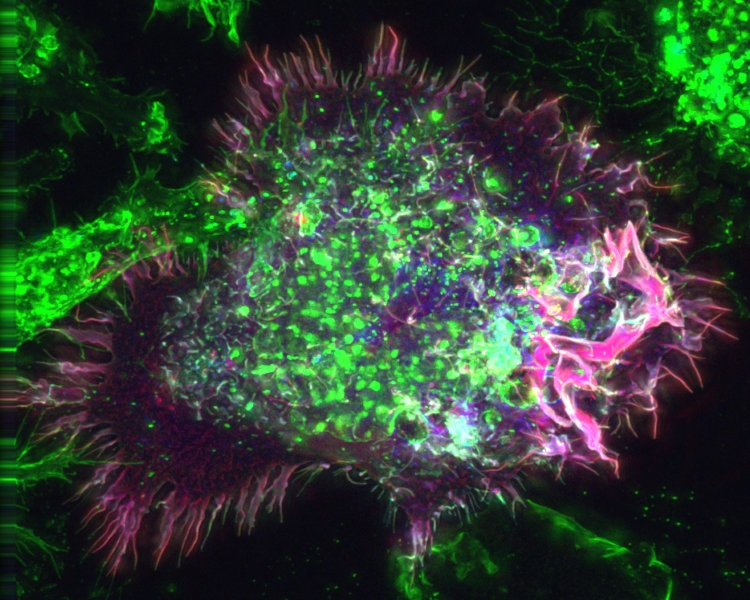
In animal model xenograft studies, the inhibitor acted synergistically with a common chemotherapy drug, cyclophosphamide, to reduce tumor growth. Thus
macropinocytosis, a rarely described form of cell death, may aid in the treatment of
cancer.
Dr Mohammad Athar, Ph.D., professor in the University of Alabama at Birmingham Department of Dermatology told
Thailand Medical News, "Understanding the signaling pathways underlying
macropinocytosis-associated cell death is an important step in developing additional effective strategies to treat neoplasms that are resistant to apoptosis induced by chemotherapy."
Typically, the inhibitor, OSI-027, affects the mTOR pathway, which plays a critical role in regulation cellular growth and metabolism. Significantly, this potent inhibitor simultaneously targets two distinct protein complexes of the mTOR pathway, mTORC1 and mTORC2. Aberrant activation of these components has been associated with many cancer types.
Normally, macropinocytosis starts with formation of ruffles on the surface of a cell that reach out from the cell membrane. These ruffles then fuse back with the cell membrane, creating a bubble that holds extracellular fluid, and the bubble moves inside the cell to become a vacuole filled with fluid. In catastrophic micropinocytosis, large numbers of these vacuoles form inside the cell and then fuse together, causing cell death.
The researchers showed that dual inhibition of the two mTORC1 and -C2 complexes was necessary for highly effective cell death through
macropinocytosis.
In earlier experiments, the researchers found that OSI-027, and a related dual inhibitor, PP242, induced extensive vacuolization in a wide range of human
cancer cell lines, including two subtypes of rhabdomyosarcoma. These vacuoles were then shown to be macropinosomes.
Xenograft animal models experiments with human rhabdomyosarcoma tumors showed that OSI-027 blocked tumor growth by inducing
macropinocytosis; furthermore, the addition of the chemotherapy agent cyclophosphamide acted synergistically to enhance efficacy of tumor size reduction.
Dr Athar and colleagues nn mechanistic studies, found that
macropinocytosis depended on activation of the MAP kinase MKK4, which was induced by the presence of reactive oxygen species. However, the full role of MKK4 is not well understood, they say.
Past work by others had shown that several specific inducers of macropinocytosis induced
macropinocytosis mainly in glioblastomas and colorectal cells. "In contrast," Athar said, "our study demonstrates that the dual inhibitors w
e tested induce catastrophic vacuolization in tumor cell lines from a wide range of organs, including skin, breast, cervix, lung and soft tissues.” The effects were much less pronounced in immortalized human keratinocytes.
Dr Athar added, "Our data reveal that therapeutic targeting of mTORC1 and mTORC2, together with standard care treatment," "may be an effective approach to block the pathogenesis of recurrent rhabdomyosarcoma and perhaps other drug-resistant invasive neoplasms of diverse tissue types as well. The underlying mechanism by which tumors become responsive to treatment involves
macropinocytosis, a unique form of cell death."
Reference: Ritesh K. Srivastava et al, Combined mTORC1/mTORC2 inhibition blocks growth and induces catastrophic macropinocytosis in cancer cells, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1911393116
