Nikhil Prasad Fact checked by:Thailand Medical News Team May 28, 2024 10 months, 5 days, 21 hours, 36 minutes ago
COVID-19 News: In a groundbreaking study, researchers from prestigious institutions across the United States have uncovered a crucial mechanism that SARS-CoV-2, the virus behind COVID-19, uses to wreak havoc on human cells. The study, involving teams from Vanderbilt University, the Icahn School of Medicine at Mount Sinai, Texas Biomedical Research Institute, and the University of Texas Southwestern Medical Center and is covered in this
COVID-19 News report, reveals how the virus's nonstructural protein 1 (Nsp1) disrupts mRNA nuclear export, a vital cellular process. This discovery not only sheds light on the virus's virulence but also opens up new avenues for antiviral drug development.
 Inhibiting mRNA Nuclear Export Increases SARS-CoV-2 Pathogenic Potential
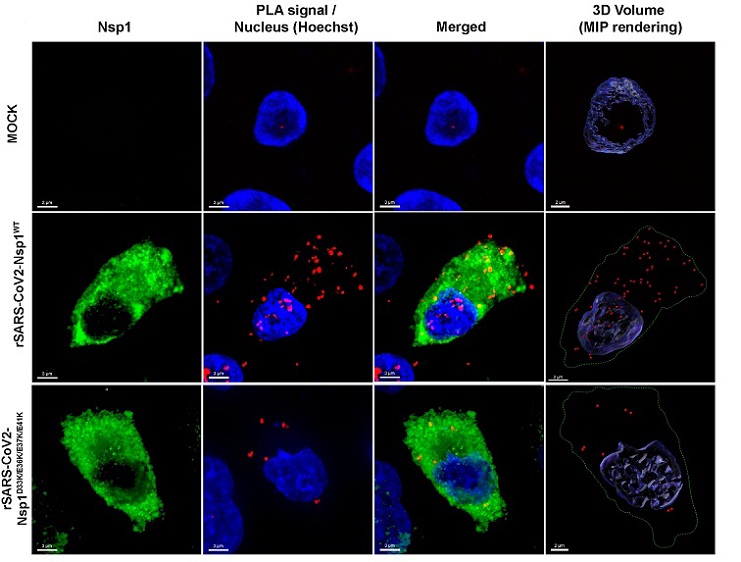
Nsp1-NXF1 interaction is mostly cytoplasmic in rSARS-CoV2WT infected cells and is decreased in rSARS-CoV2D33K/E36K/E37K/E41K infected cells. (A) A549-ACE2 cells were mock infected or infected with rSARS-CoV2WT or rSARS-CoV2D33K/E36K/E37K/E41K. After 12 h, cells were subjected to PLA to detect the interaction between Nsp1 and NXF1 proteins in situ. The interaction by PLA is detected by fluorescent probes (red dots; λem = 624 nm, TRITC filter). Immunofluorescence staining of Nsp1 is shown in green. Hoechst staining labels the nuclei (blue). (Scale bar: 3 μm). The panels on the Right are three-dimensional projections of chromatin merged surface with the PLA signals detecting the NXF1–Nsp1 complexes. Cell boundaries are marked by green-dotted lines.
The Study's Significance
Inhibiting mRNA Nuclear Export Increases SARS-CoV-2 Pathogenic Potential
Nsp1-NXF1 interaction is mostly cytoplasmic in rSARS-CoV2WT infected cells and is decreased in rSARS-CoV2D33K/E36K/E37K/E41K infected cells. (A) A549-ACE2 cells were mock infected or infected with rSARS-CoV2WT or rSARS-CoV2D33K/E36K/E37K/E41K. After 12 h, cells were subjected to PLA to detect the interaction between Nsp1 and NXF1 proteins in situ. The interaction by PLA is detected by fluorescent probes (red dots; λem = 624 nm, TRITC filter). Immunofluorescence staining of Nsp1 is shown in green. Hoechst staining labels the nuclei (blue). (Scale bar: 3 μm). The panels on the Right are three-dimensional projections of chromatin merged surface with the PLA signals detecting the NXF1–Nsp1 complexes. Cell boundaries are marked by green-dotted lines.
The Study's Significance
The importance of this study lies in its detailed exploration of how inhibiting mRNA nuclear export can enhance the pathogenesis of SARS-CoV-2. By focusing on the interaction between the virus's Nsp1 protein and the mRNA export receptor NXF1, the researchers were able to pinpoint the exact mechanisms through which the virus halts the export of mRNA from the nucleus to the cytoplasm. This blockade is crucial for the virus's replication and ability to cause disease. Furthermore, the study introduces a mutant version of SARS-CoV-2 that cannot inhibit mRNA export, resulting in a less pathogenic virus. This mutant provides a valuable tool for understanding and potentially mitigating the virus's impact.
Understanding mRNA Nuclear Export
mRNA nuclear export is a critical step in the process of gene expression. In eukaryotic cells, mRNA molecules must travel from the nucleus, where they are synthesized, to the cytoplasm, where they are translated into proteins. This journey is facilitated by the mRNA export receptor NXF1, which forms a complex with NXT1 to transport mRNA through the nuclear pore complex (NPC). The proper functioning of this pathway is essential for cellular health and response to viral infections.
Nsp1: A Multifunctional Virulence Factor
SARS-CoV-2's Nsp1 protein is a key player in its strategy to hijack host cellular machi
nery. Nsp1 targets multiple pathways to suppress host gene expression and immune responses. Previous research has shown that Nsp1 can bind to the ribosome's 40S subunit, plugging the mRNA entry channel and halting protein translation. However, Nsp1 also plays a role in inducing mRNA decay and, as this new study highlights, inhibiting mRNA nuclear export.
The Acidic Patch: A Key Interaction Surface
Through a combination of structural biology and mutagenesis, the researchers identified an acidic patch on the N-terminal domain of Nsp1 that is crucial for its interaction with the NXF1-NXT1 complex. Mutations in this acidic patch led to a separation-of-function mutant of Nsp1 that could still inhibit translation but lost its ability to block mRNA export. This separation allowed the researchers to study the specific contributions of mRNA export inhibition to viral replication and pathogenesis.
Creating a Less Pathogenic Virus
Armed with this knowledge, the team engineered a recombinant SARS-CoV-2 virus with mutations in the Nsp1 acidic patch. This mutant virus, unable to inhibit mRNA export, showed significantly reduced replication and pathogenicity in both cellular and animal models. Infected cells and mice displayed lower levels of viral proteins and milder symptoms, respectively. This demonstrates the pivotal role of Nsp1-mediated mRNA export inhibition in the virus's ability to cause severe disease.
Implications for Antiviral Development
The study's findings offer promising new targets for antiviral drug development. By focusing on the Nsp1-NXF1 interaction, researchers can develop compounds that specifically disrupt this pathway, potentially reducing the virus's ability to replicate and cause disease. Such targeted therapies could complement existing treatments and enhance the overall response to COVID-19 and future coronavirus outbreaks.
A Broader Perspective on Viral Strategies
This research also contributes to a broader understanding of viral strategies for hijacking host cell machinery. Inhibition of host gene expression is a common tactic employed by diverse viruses, including influenza A, which uses a similar approach to block mRNA export. By comparing these mechanisms, scientists can uncover universal principles of viral pathogenesis and devise broad-spectrum antiviral strategies.
Future Directions
Moving forward, the research team aims to further explore the molecular interactions between Nsp1 and NXF1, as well as other host factors involved in mRNA export. Additionally, they plan to test potential inhibitors that can disrupt the Nsp1-NXF1 interaction in laboratory and clinical settings. By continuing to unravel the complex strategies employed by SARS-CoV-2, scientists hope to stay one step ahead of the virus and enhance our ability to combat it effectively.
Conclusion
The study findings represent a significant advance in our understanding of SARS-CoV-2's virulence mechanisms. By elucidating how Nsp1 inhibits mRNA nuclear export, the study not only provides a detailed molecular mechanism but also highlights potential targets for antiviral therapy. As the world continues to grapple with the impacts of COVID-19, such insights are invaluable for developing more effective treatments and preventing future pandemics.
The study findings were published in the peer reviewed journal: PNAS.
https://www.pnas.org/doi/10.1073/pnas.2314166121
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/study-finds-that-sars-cov-2-nsp15-suppresses-type-i-interferon-production-by-inhibiting-irf3-phosphorylation-and-nuclear-translocation
https://www.thailandmedical.news/news/breaking-sars-cov-2-nucleocytoplasmic-shuttling,-nuclear-localization-and-production-of-viral-host-chimeric-proteins-causes-long-covid-issues
https://www.thailandmedical.news/news/covid-19-research-chinese-study-finds-that-sars-cov-2-nsp15-protein-suppresses-type-1-interferon-production
