Interferon-γ Can Be Used An Antiviral Against SARS-CoV-2 As It Inhibits ORF6 Protein
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 11, 2024 1 year, 2 months, 5 days, 2 hours, 4 minutes ago
COVID-19 News: The emergence of the novel coronavirus SARS-CoV-2 in late 2019 led to a global pandemic, prompting the development of vaccines and antiviral drugs. While significant progress has been made, the ongoing challenges, including asymptomatic cases and the rise of new variants, emphasize the need for innovative therapeutic strategies. Among the various proteins encoded by SARS-CoV-2, ORF6 stands out for its role in inhibiting the host's innate immune response and disrupting mRNA transport, making it a potential target for antiviral interventions. A new study covered in this
COVID-19 News report, conducted by researchers from Bulgarian Academy of Sciences, Sofia-Bulgaria and Sofia University “St. Kliment Ohridski”-Bulgaria have found that interferon-γ could be used to inhibit the Orf6 proteins of SARS-CoV-2.
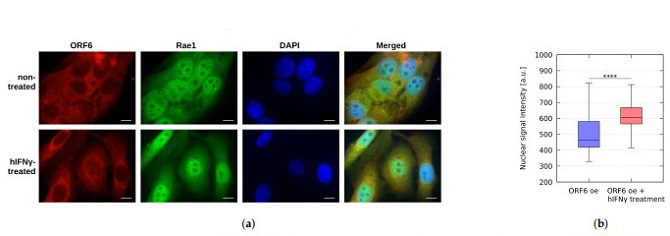 RAE1 localization in ORF6 overexpressing cells upon hIFNγ treatment. (a) Non-treated and hIFNγ-treated cells, overexpressing ORF6, were stained with antibodies against RAE1 and ORF6. Representative images are shown. Scale bar is 5 µm. (b) The fluorescence intensity of RAE1 (in the green channel) was analyzed for each cell using the ImageJ version 1.54h. software in ORF6 oe cells and ORF6 oe cells upon hIFNγ treatment. The difference is statistically significant with a **** p-value < 0.0001, estimated using Student’s test for at least three independent experiments.
SARS-CoV-2 Genome and Protein Overview
RAE1 localization in ORF6 overexpressing cells upon hIFNγ treatment. (a) Non-treated and hIFNγ-treated cells, overexpressing ORF6, were stained with antibodies against RAE1 and ORF6. Representative images are shown. Scale bar is 5 µm. (b) The fluorescence intensity of RAE1 (in the green channel) was analyzed for each cell using the ImageJ version 1.54h. software in ORF6 oe cells and ORF6 oe cells upon hIFNγ treatment. The difference is statistically significant with a **** p-value < 0.0001, estimated using Student’s test for at least three independent experiments.
SARS-CoV-2 Genome and Protein Overview
SARS-CoV-2 possesses a large positive-sense RNA genome of approximately 30 kilobases, comprising 14 open reading frames (ORFs) translated into 29 proteins. These include structural proteins, non-structural proteins (NSP1-16), and accessory proteins (ORF3a, 3b, 6, 7a, 7b, 8, 9b, 9c, and 10). While structural and non-structural proteins share high homology with SARS-CoV, the accessory proteins, particularly ORF6, exhibit lower homology. The ORF6 protein, consisting of 61 amino acids, is noteworthy for its amphipathic N-terminal and highly charged C-terminal regions.
ORF6's Impact on Host Immunity
Extensive research has revealed that ORF6 plays a pivotal role in suppressing the host's primary immune response. It disrupts bidirectional nucleo-cytoplasmic trafficking by interacting with the ribonucleic acid export factor 1 (RAE1) and nucleoporin 98 (NUP98), inhibiting the transport of essential mRNAs from the nucleus to the cytoplasm. Additionally, ORF6 has been identified as a potent antagonist of type I interferon (IFN) pathways, demonstrating the highest toxicity among SARS-CoV-2 proteins.
ORF6-RAE1 Interaction and Implications
The interaction between ORF6 and RAE1 leads to the immobilization of RAE1 on cytoplasmic membranes, hindering mRNA export and negatively affecting cellular genome stability. This disruption of mRNA transport also results in the accumulation of RN
A-DNA hybrids, contributing to genomic instability. Furthermore, ORF6 has been linked to the breakdown of the DNA damage response kinase CHK1, inducing DNA damage, pro-inflammatory pathways, and cellular senescence.
Current Therapeutic Gap and Proposed Strategy
Despite the critical role of ORF6 in SARS-CoV-2 pathogenicity, specific and effective inhibitors for this protein have not been reported to date. This study presents findings from computational and laboratory studies suggesting interferon-γ (hIFNγ) as a promising inhibitor of ORF6. Computational modeling demonstrates the high affinity of hIFNγ for the C-terminus of ORF6, suggesting its potential to disrupt the protein's interactions with RAE1.
Experimental Validation of hIFNγ as an ORF6 Inhibitor
In vitro studies provide experimental evidence supporting the inhibitory effects of hIFNγ on ORF6. Treatment with hIFNγ leads to a shift in the subcellular localization of RAE1, restoring its predominantly nuclear presence and facilitating mRNA export from the nucleus to the cytoplasm. The expression of green fluorescent protein (GFP) in cells overexpressing ORF6 is significantly reinstated upon hIFNγ treatment, indicating the restoration of mRNA trafficking.
Effect of hIFNγ on mRNA Transport and Genome Stability
The ability of hIFNγ to unblock mRNA trafficking is further supported by the observed reduction in accumulated RNA-DNA hybrids. The restoration of replication fork rates in cells overexpressing ORF6, along with the prevention of R-loop formation, underscores the potential of hIFNγ to counteract the detrimental effects of ORF6 on cellular proliferation and genome stability.
Implications for COVID-19 Treatment
The findings presented in this study propose interferon-γ as a promising therapeutic agent against SARS-CoV-2 by targeting the toxic effects of the ORF6 protein. While the complexities of hIFNγ's dual role in the immune system require further exploration, its potential to inhibit ORF6 offers a novel avenue for developing antiviral strategies. The observed positive outcomes in treating patients with moderate coronavirus infection using recombinant hIFNγ highlight the therapeutic potential of this cytokine.
Conclusion
In conclusion, the research outlined in this article sheds light on the inhibitory effects of interferon-γ on the SARS-CoV-2 accessory protein ORF6. The computational modeling and experimental validation provide a comprehensive understanding of the potential therapeutic role of hIFNγ in restoring host immune responses and mitigating the viral pathogenicity associated with ORF6. Further investigations into the timing, dosage, and duration of hIFNγ application are warranted to optimize its effectiveness as a potential antiviral against SARS-CoV-2.
The study findings were published in the peer reviewed International Journal of Molecular Sciences.
https://www.mdpi.com/1422-0067/25/4/2155
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
