Interleukin-1 Beta Inhibits SARS-CoV-2 Spread By Preventing Cell Fusion Through Actin Bundle
Nikhil Prasad Fact checked by:Thailand Medical News Team May 21, 2024 1 year, 7 months, 1 week, 1 day, 4 hours, 39 minutes ago
COVID-19 News: The COVID-19 pandemic, driven by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a profound impact on global health. With over 755 million infections and more than 6.8 million deaths worldwide as reported in various
COVID-19 News coverages, the search for effective treatments and preventive strategies remains crucial. SARS-CoV-2 variants, such as Alpha, Beta, Delta, and Omicron, continue to challenge our ability to control the virus due to their increased transmissibility and immune escape capabilities. Understanding the host responses to SARS-CoV-2 infection is essential for developing new strategies to combat the virus.
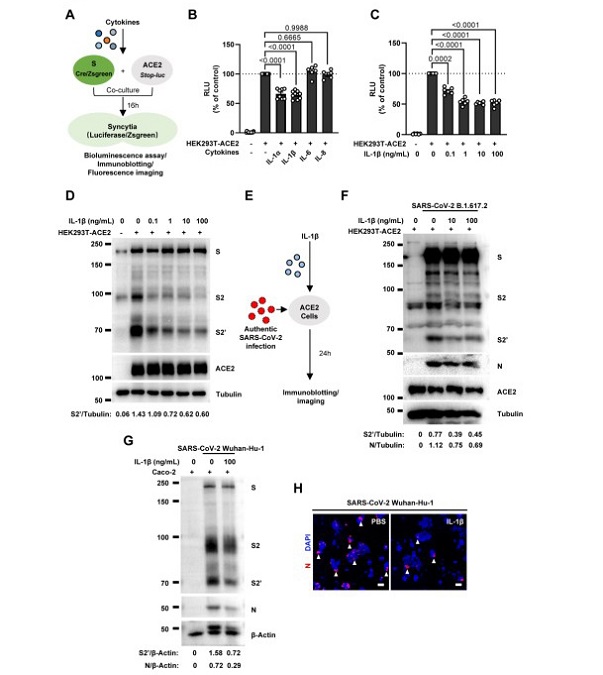 IL-1β inhibits SARS-CoV-2 induced cell-cell fusion.
(A) Schematics of the cell-cell fusion model used to quantify spike-mediated syncytium formationupon treatment with different cytokines. (B) Luciferase activity (RLU) measured from HEK293T cell ysates collected from different cytokines treated HEK293T-S and HEK293T-ACE2. IL-1α (10 ng/mL), IL-1β (1 ng/mL), IL-6 (100 ng/mL) or IL-8 (100 ng/mL) were added into the cell-cell fusion system. Data are representative of six individual repeats and displayed as individual points with mean ± standard error of the mean (SEM). (C) Luciferase activity (RLU) measured from HEK293T cell lysates collected from different concentrations of IL833 1β treated HEK293T-S and HEK293T-ACE2 for 16 hours. Data are representative of six individual repeats and displayed as individual points with mean ± standard error of the mean (SEM). (D) Immunoblots showing full-length spike, S2, cleaved S2’ and ACE2 collected from different concentrations of IL-1β treated HEK293T-S and HEK293T-ACE2 for 16 hours. Blots are representative of three independent experiments. Numbers below the blots indicated the ratio of S2’ versus Tubulin. (E) Schematic presentation of IL-1β pre-treatment on authentic SARS-CoV-2 infected cells. (F) Immunoblots of Delta SARS-CoV-2 S, S2, cleaved S2’, N and ACE2 proteins collected from HEK293T-ACE2 cells 24 hpi as described in (E). Blots are representative of three individual experiments. Numbers below the blots indicated the ratio of S2’ or N versus Tubulin. (G) Immunoblots of WT SARS-CoV-2 S, S2, cleaved S2’ and N protein collected from Caco-2 cells 24 hpi as described in (E). Blots are representative of three individual experiments. Numbers below the blots indicated the ratio of S2’ or N versus β-Actin. (H) Immunofluorescent images showing morphology of SARS-CoV-2 infected Caco-2 cells pre849 treated with or without IL-1β. Anti-SARS-CoV-2 N was stained with Alexa fluor 555 and nuclei were counterstained with DAPI respectively.
The Role of Cell-Cell Fusion in Viral Spread
IL-1β inhibits SARS-CoV-2 induced cell-cell fusion.
(A) Schematics of the cell-cell fusion model used to quantify spike-mediated syncytium formationupon treatment with different cytokines. (B) Luciferase activity (RLU) measured from HEK293T cell ysates collected from different cytokines treated HEK293T-S and HEK293T-ACE2. IL-1α (10 ng/mL), IL-1β (1 ng/mL), IL-6 (100 ng/mL) or IL-8 (100 ng/mL) were added into the cell-cell fusion system. Data are representative of six individual repeats and displayed as individual points with mean ± standard error of the mean (SEM). (C) Luciferase activity (RLU) measured from HEK293T cell lysates collected from different concentrations of IL833 1β treated HEK293T-S and HEK293T-ACE2 for 16 hours. Data are representative of six individual repeats and displayed as individual points with mean ± standard error of the mean (SEM). (D) Immunoblots showing full-length spike, S2, cleaved S2’ and ACE2 collected from different concentrations of IL-1β treated HEK293T-S and HEK293T-ACE2 for 16 hours. Blots are representative of three independent experiments. Numbers below the blots indicated the ratio of S2’ versus Tubulin. (E) Schematic presentation of IL-1β pre-treatment on authentic SARS-CoV-2 infected cells. (F) Immunoblots of Delta SARS-CoV-2 S, S2, cleaved S2’, N and ACE2 proteins collected from HEK293T-ACE2 cells 24 hpi as described in (E). Blots are representative of three individual experiments. Numbers below the blots indicated the ratio of S2’ or N versus Tubulin. (G) Immunoblots of WT SARS-CoV-2 S, S2, cleaved S2’ and N protein collected from Caco-2 cells 24 hpi as described in (E). Blots are representative of three individual experiments. Numbers below the blots indicated the ratio of S2’ or N versus β-Actin. (H) Immunofluorescent images showing morphology of SARS-CoV-2 infected Caco-2 cells pre849 treated with or without IL-1β. Anti-SARS-CoV-2 N was stained with Alexa fluor 555 and nuclei were counterstained with DAPI respectively.
The Role of Cell-Cell Fusion in Viral Spread
SARS-CoV-2 infection leads to cell-cell fusion, also known as syncytia formation, in various cell types including lung epithelial cells, neurons, and glia. This fusion process facilitates vi
ral transmission and contributes to the pathogenicity of the virus. Syncytia formation helps the virus evade extracellular neutralizing antibodies, making it harder to control the infection. Observations from lung autopsies of deceased COVID-19 patients have shown long-term persistence of viral RNA and syncytia formation, which may be linked to prolonged viral clearance and long COVID symptoms. Therefore, inhibiting syncytia formation is critical for effective viral clearance and controlling transmission.
Innate Immune Responses and Cytokines
Innate immune responses are the first line of defense against viral infections. Various cells, including lung epithelial cells and innate immune cells like macrophages and monocytes, play crucial roles in responding to SARS-CoV-2 infection. These cells release pro-inflammatory cytokines, which are essential for viral containment. Among these cytokines, interleukin-1β (IL-1β) has been identified as a key factor in inhibiting SARS-CoV-2-induced syncytia formation.
Discovery of IL-1β's Antiviral Function
Through extensive research using human monocytes and cytokine screening, scientists discovered that IL-1β significantly inhibits the formation of syncytia induced by SARS-CoV-2. IL-1β achieves this by activating the RhoA/ROCK signaling pathway through a non-canonical IL-1 receptor-dependent mechanism. This activation leads to the formation of actin bundles at cell-cell junctions, preventing the fusion of SARS-CoV-2-infected cells with neighboring cells. This mechanism effectively restricts viral transmission.
Experimental Validation in Mice
The antiviral function of IL-1β was further confirmed through in vivo experiments in mice. When IL-1β was administered to mice infected with SARS-CoV-2, the spread of the virus within the lung epithelia was significantly restricted. These findings highlight the potential of IL-1β as an antiviral agent that could be harnessed to control SARS-CoV-2 transmission.
Mechanistic Insights
-Activation of RhoA/ROCK Pathway
IL-1β activates the RhoA/ROCK signaling pathway, which is crucial for the formation of actin bundles at cell-cell junctions. This pathway involves several key molecules, including MyD88, IRAK, and TRAF6. Inhibition or knockout of these molecules prevents IL-1β from activating RhoA and forming actin bundles, thereby allowing syncytia formation to proceed. This indicates that the IL-1β-mediated inhibition of cell-cell fusion is dependent on the intact RhoA/ROCK signaling pathway.
-Independent of TAK1-IKKβ-NF-κB Signaling
Interestingly, the study found that the inhibition of syncytia formation by IL-1β is independent of the TAK1-IKKβ-NF-κB signaling cascade. Even when these pathways were inhibited, IL-1β continued to reduce syncytia formation, indicating a specific and direct role of the RhoA/ROCK pathway in this process.
Broader Implications - Inhibition of Other Coronaviruses
IL-1β's ability to inhibit cell-cell fusion is not limited to SARS-CoV-2. The study demonstrated that IL-1β could also prevent syncytia formation induced by other coronaviruses such as SARS-CoV and MERS-CoV. This broad-spectrum activity underscores the potential of IL-1β as a universal antiviral agent against various coronaviruses.
Therapeutic Potential
The findings from this study suggest that targeting the RhoA/ROCK signaling pathway could be a viable therapeutic strategy to control viral transmission. By inducing actin bundle formation at cell-cell junctions, IL-1β effectively creates physical barriers that prevent viral spread. This approach could complement existing treatments and enhance the efficacy of antiviral therapies.
Conclusion
The study provides compelling evidence that IL-1β plays a critical role in preventing SARS-CoV-2-induced syncytia formation and subsequent viral transmission. By activating the RhoA/ROCK signaling pathway, IL-1β induces the formation of actin bundles at cell-cell junctions, creating a barrier to viral spread. These findings highlight the importance of innate immune factors, such as IL-1β, in controlling viral infections and offer new avenues for therapeutic development. As we continue to battle the COVID-19 pandemic and prepare for potential future outbreaks, harnessing the power of IL-1β and similar cytokines could prove invaluable in our efforts to protect global health.
The study findings by researchers from the University of Chinese Academy of Sciences, Shanghai-China, Shanghai Municipal Center for Disease Control and Prevention-China, The First Affiliated Hospital of Guangzhou Medical University-China, Soochow University, Jiangsu-China and Shanghai Blood Center-China were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.05.16.594569v1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
