Source: Thailand Medical News Dec 07, 2019 5 years, 11 months, 6 days, 13 hours, 44 minutes ago
In Asian populations, despite
biliary tract cancer being a type of
cancer that is considered rare globally, its growing incidence rate is becoming a worrisome trend. In countries like Thailand, Cambodia, Vietnam, Myanmar, China, Korea and Japan, the incidence of
bile duct cancer has been rising at an exponential rate. Currently
Thailand* has the highest incidence rate in the world with an estimated rate of 113 per 100,000 males and 50 per 100,000 for females. Its attributed to liverfluke infections which is contracted by eating raw fish, raw prawns, raw meats etc.
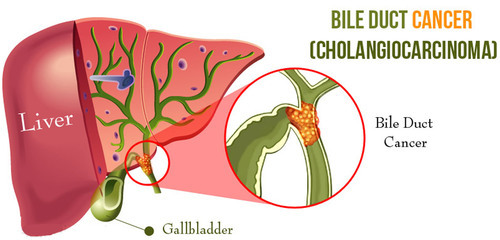 Bile duct cancer
Bile duct cancer is a type of
cancer that occurs in the bile ducts, which transport bile from the liver to the duodenum. Patients with one form of this
cancer, called intrahepatic
cholangiocarcinoma (ICC), have very poor outcomes and rarely survive beyond five years after their diagnosis.
Researchers at Tokyo Medical and Dental University (TMDU) have uncovered a protein that may eventually help doctors predict which patients with ICC will have the worst outcomes and that may someday lead to more effective treatments for ICC.
New studies have found that ICC tumors tend to have genetic mutations leading to the loss of a protein called ARID1A (AT-rich interactive domain-containing protein 1A). However, the exact role of the protein in ICC was unknown.
Dr Jun Yoshino, lead author of the study told
Thailand Medical News, "ARID1A is known to take part in complexes that control gene expression. This hinted that loss of ARID1A could play a role in ICC by causing certain genes to turn on or off. However, it wasn't clear which genes might be affected or how their expression might impact the ICC disease course."
To address this question, the researchers looked at ICC cell lines ie cells that are grown in dishes and commonly used as a model for ICC tumors. Using the genome editing technique CRISPR/Cas9, the researchers deleted the gene coding for ARID1A, resulting in "knock-out" cells that mimic patients who have ARID1A-negative ICC tumors. The team discovered that when they removed ARID1A, the cells became especially malignant.
Dr. Yoshino explained, "We found that ARID1A suppresses a stemness gene ALDH1A1 implicated in other
cancers. Removing ARID1A causes the gene to become highly overactive in ICC cells. We found that when this cancer stemness gene is overactive, it causes the cells to act much more aggressively. For example, the cells can migrate and invade neighboring groups of cells, a behavior that is very common in highly-active cancers."
The highly aggressive behavior of the cells suggested that ARID1A-negative ICC might represent a distinct form of the
cancer, perhaps with a distinct prognosis. To confirm this suspicion, the team measured levels of ARID1A in tissue samples from patients with ICC, and then followed their medical history to see how well the patient
s eventually fared. They found that patients who were ARID1A-negative indeed had much poorer outcomes: five years after their diagnosis, less than 20% of ARID1A-negative patients had survived, compared with over 50% of ARID1A-positive patients.
Dr Shinji Tanaka, corresponding author of the study commented, "ARID1A-negative patients are more likely to have high levels of a tumorigenic gene, which in turn enhances the malignancy of their cancer. This has important medical implications for patients with ICC. For one, it may be possible to use ARID1A as a biomarker to help physicians predict the prognosis of their patients. What's more, further research into how ARID1A functions could someday lead to more sophisticated treatment methods for this form of the disease."
Reference : Jun Yoshino et al, Loss of ARID1A induces a stemness gene ALDH1A1 expression with histone acetylation in the malignant subtype of cholangiocarcinoma, Carcinogenesis (2019). DOI: 10.1093/carcin/bgz179
Note* : Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations
Lorena Marcano-Bonilla, Essa A. Mohamed, Taofic Mounajjed, Lewis R. Roberts doi: 10.21037/cco.2016.10.09 https://cco.amegroups.com/article/view/12259/12686
