Japanese Researchers Discover The Protective Power Of Supersulphides In Viral And Chronic Lung Diseases, Including COVID-19
Thailand Medical News Team Aug 08, 2023 1 year, 8 months, 1 week, 6 days, 1 hour, 44 minutes ago
COVID-19 Research: In a new study conducted at the Tohoku University Graduate School of Medicine in Japan, researchers have unveiled a remarkable discovery that could revolutionize our understanding of airway protection in the face of viral and chronic lung diseases, with a specific focus on conditions like COVID-19.
 Credit: NiseriN/iStock/Getty Images
Credit: NiseriN/iStock/Getty Images
The findings shed light on the role of supersulphides, a group of inorganic and organic sulphides with unique physiological functions, and their potential as a therapeutic approach to safeguarding respiratory health.
Supersulphides have long been recognized for their diverse and intricate functions involving sulphur catenation, a process mediated by mitochondrial cysteinyl-tRNA synthetase (CARS2), which acts as the principal cysteine persulphide synthase (CPERS). This recent research endeavors to unravel the protective capabilities of supersulphides in the context of various airway challenges, ranging from viral infections like influenza and COVID-19 to the complexities of aging lungs and chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF).
A novel methodology known as "breath supersulphur-omics" was developed, enabling scientists to assess the levels of exhaled supersulphides in individuals afflicted with COVID-19 infection. The study extended its investigation to a hamster model of SARS-CoV-2 infection, revealing a significant increase in exhaled supersulphides, thereby suggesting their potential as biomarkers for COVID-19.
One of the most striking outcomes of this
COVID-19 Research is the demonstration of the role of endogenous supersulphide production in mitigating lung damage caused by oxidative stress and inflammation. In mouse models of COPD, IPF, and aging, the presence of supersulphides originating from CARS2/CPERS was shown to ameliorate the harmful effects of these conditions. Furthermore, exogenous administration of the supersulphide donor, glutathione trisulphide, displayed similar protective effects.
These findings highlight the therapeutic potential of targeting supersulphides to combat a range of respiratory ailments.
Supersulphides, which encompass hydropersulphides (RSSH) and polymeric sulphur species with sulphur catenation (RSSnR, n > 1, R = hydrogen or alkyl, or cyclized polysulphides), have gained recognition for their abundance in various mammalian cells and tissues.
These compounds possess unique redox-active properties, making them more reactive and potent compared to other simple thiols and disulphides.
Interestingly, hydrogen sulphide (H2S), previously considered a small signaling molecule, was revealed to be intricately linked to the presence and activities of supersulphides. While H2S has been implicated in redox signaling, many biological effects attributed to H2S are actually the result of supersulphides' actions.
Oxidative stress has long been implicated in the onset and progression of various respiratory conditions, including influenza, COVID-
19, COPD, emphysema, IPF, and lung aging.
The study uncovered a reduction in endogenous supersulphide production in conditions like COPD and related inflammatory airway diseases. This observation led to the hypothesis that supersulphides could play a crucial protective role in mitigating oxidative stress during viral and inflammatory diseases affecting the airways.
The study focused on the pivotal role of cysteinyl-tRNA synthetases (CARS) and their function as cysteine persulphide synthases (CPERS) in supersulphide production. Specifically, CARS2, a mitochondrial isoform of CARS, was found to contribute to mitochondrial biogenesis and bioenergetics through the production of CysSSH, a type of supersulphide. This mechanism was identified as a potential protective avenue against a spectrum of airway diseases.
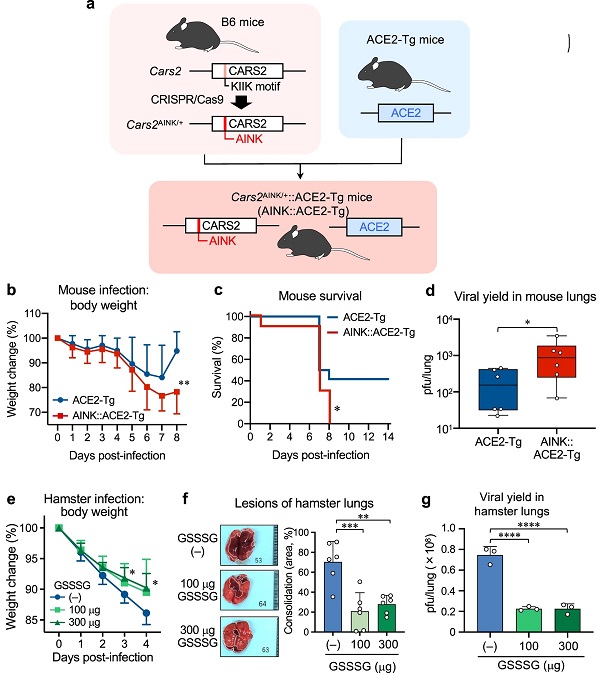 a Schematic drawing of the method for generation of ACE2-Tg::CPERS-deficient (AINK::ACE2-Tg) mice. Cars2AINK/+ mice were produced via CRISPR-Cas9 system and crossed with ACE2-Tg mice to generate AINK::ACE2-Tg mice. Supplementary Fig. 7 shows genomic modification at Cars2 locus by the CRISPR-Cas9 system. b–d SARS-CoV-2 infection in mice. b, Mean body weight changes (95%, CI) of SARS-CoV-2-infected ACE2-Tg (n = 12) and AINK::ACE2-Tg mice (n = 10). All mice were infected intratracheally (i.t.) with 100 pfu (per 50 μl) of SARS-CoV-2; body weight was monitored until day 8 post-infection. P = 0.0045. c Kaplan–Meier survival curves for ACE2-Tg (n = 12) and AINK::ACE2-Tg mice (n = 10) infected with SARS-CoV-2; survival was monitored until day 14 post-infection. P = 0.035. d Viral yield in the infected lungs (homogenates) at 4 days post-infection. The viral titers were determined by plaque-forming assay and are expressed as a plaque-forming unit (pfu)/lung. P = 0.048. e–g SARS-CoV-2 infection in hamsters. Hamsters were infected i.t. with 6 × 106 pfu (120 μl) of SARS-CoV-2; with simultaneous i.t. administration of 100 or 300 μg GSSSG. The hamsters were subsequently administered intraperitoneally (i.p.) 500 or 1000 μg GSSSG daily from day 1 to day 4. e Mean body weight changes (95% CI) in SARS-CoV-2-infected hamster that were given PBS (n = 6) or GSSSG (n = 6), as being monitored until day 4 post-infection. P = 0.035 (3 days) and 0.020 (4 days). f Semi-quantitative measurement of the area of consolidation (pathological lesion) of SARS-CoV-2-infected hamster lungs with or without GSSSG treatment (n = 6). P = 0.0004 and 0.0015. g The amounts (pfu) of virus yielded in the lungs of hamsters treated or untreated with GSSSG after infection, as assessed by the plaque-forming assay at 4 days post-infection. P < 0.0001 in both. Data are means ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
a Schematic drawing of the method for generation of ACE2-Tg::CPERS-deficient (AINK::ACE2-Tg) mice. Cars2AINK/+ mice were produced via CRISPR-Cas9 system and crossed with ACE2-Tg mice to generate AINK::ACE2-Tg mice. Supplementary Fig. 7 shows genomic modification at Cars2 locus by the CRISPR-Cas9 system. b–d SARS-CoV-2 infection in mice. b, Mean body weight changes (95%, CI) of SARS-CoV-2-infected ACE2-Tg (n = 12) and AINK::ACE2-Tg mice (n = 10). All mice were infected intratracheally (i.t.) with 100 pfu (per 50 μl) of SARS-CoV-2; body weight was monitored until day 8 post-infection. P = 0.0045. c Kaplan–Meier survival curves for ACE2-Tg (n = 12) and AINK::ACE2-Tg mice (n = 10) infected with SARS-CoV-2; survival was monitored until day 14 post-infection. P = 0.035. d Viral yield in the infected lungs (homogenates) at 4 days post-infection. The viral titers were determined by plaque-forming assay and are expressed as a plaque-forming unit (pfu)/lung. P = 0.048. e–g SARS-CoV-2 infection in hamsters. Hamsters were infected i.t. with 6 × 106 pfu (120 μl) of SARS-CoV-2; with simultaneous i.t. administration of 100 or 300 μg GSSSG. The hamsters were subsequently administered intraperitoneally (i.p.) 500 or 1000 μg GSSSG daily from day 1 to day 4. e Mean body weight changes (95% CI) in SARS-CoV-2-infected hamster that were given PBS (n = 6) or GSSSG (n = 6), as being monitored until day 4 post-infection. P = 0.035 (3 days) and 0.020 (4 days). f Semi-quantitative measurement of the area of consolidation (pathological lesion) of SARS-CoV-2-infected hamster lungs with or without GSSSG treatment (n = 6). P = 0.0004 and 0.0015. g The amounts (pfu) of virus yielded in the lungs of hamsters treated or untreated with GSSSG after infection, as assessed by the plaque-forming assay at 4 days post-infection. P < 0.0001 in both. Data are means ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
The research also unveiled the potential of exogenously administered supersulphides, particularly the synthetic GSSSG (glutathione trisulphide), to exhibit antiviral properties and improvements in models of COPD and IPF. Given the heightened risk of severe COVID-19 outcomes in individuals with COPD and the serious complications associated with IPF, the potential therapeutic application of GSSSG represents a significant advancement.
The interaction between supersulphides and viral proteins, such as the envelope protein HA in influenza viruses and the S protein in SARS-CoV-2, provides insight into their direct antiviral effects. Supersulphides were found to induce structural alterations in viral proteins, thereby inactivating viruses and reducing their ability to attach to host cells. This discovery has broad implications for the potential of supersulphides as innate antiviral agents against a variety of viruses.
Furthermore, the study explored the immunomodulatory effects of supersulphides, highlighting their ability to regulate immune responses and suppress inflammatory reactions. The potential to ameliorate the cytokine storm associated with conditions like COVID-19 is a particularly noteworthy finding.
The culmination of this research underscores the critical role of CARS2/CPERS-mediated supersulphide production in preserving respiratory health. Moreover, the identification of supersulphides' protective effects against viral infections, chronic lung diseases, and lung aging has far-reaching implications for the development of innovative therapeutic strategies.
The study findings were published in the peer reviewed journal: Nature Communications.
https://www.nature.com/articles/s41467-023-40182-4
For the latest
COVID-19 Research updates, keep on logging to Thailand Medical News.

