Key positive residues in SARS-CoV-2 fusion domain are crucial for membrane fusion
Nikhil Prasad Fact checked by:Thailand Medical News Team Jul 13, 2024 1 year, 5 months, 1 week, 1 day, 6 hours, 32 minutes ago
COVID-19 News: In the fight against COVID-19, understanding how the virus enters and infects human cells is crucial. Researchers from the University of Maryland-USA, have made significant strides in this area. This
COVID-19 News report delves into their recent findings on the SARS-CoV-2 fusion domain (FD) and its role in membrane fusion, a key step in the viral infection process.
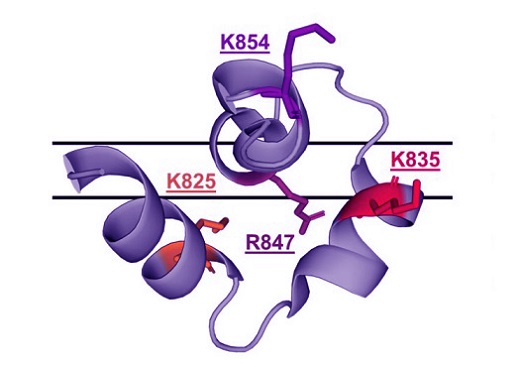 The structure of the SARS-COV-2 Fusion Domain embedded in a DMPC:DH7 PC bicelle(PDB:7MY8).
Unveiling the Fusion Domain's Role
The structure of the SARS-COV-2 Fusion Domain embedded in a DMPC:DH7 PC bicelle(PDB:7MY8).
Unveiling the Fusion Domain's Role
SARS-CoV-2, the virus responsible for COVID-19, is highly infectious. Despite extensive research, certain aspects of its viral lifecycle remain unclear, especially the process of membrane fusion. Membrane fusion is initiated by the fusion domain (FD) of the virus, which includes a fusion peptide (FP) and a fusion loop (FL). These components work together to disturb the target cell's lipid membrane, reducing the energy required for the virus to fuse with and enter the cell.
The Focus on Positive Residues
The researchers focused on four basic (positively charged) residues within the FD: K825, K835, R847, and K854. Using a mutagenesis-based approach, they investigated the roles of these residues through an in vitro fusion assay and advanced NMR techniques. The study revealed that each of these residues plays a specific role in the fusion mechanism. Intriguingly, a mutation at K825 (K825A) led to increased fusogenicity (the ability to fuse with the target cell membrane), highlighting its critical role in the fusion process.
Experimental Approach and Findings
In Vitro Fusion Assays
The team created alanine and charge-conserving mutants of the FD's basic residues. They used an in vitro fusion assay with membranes containing specific lipids to measure the impact of these mutations on fusion. The results showed that K825A led to a gain in function, while other mutations, particularly those involving R847, resulted in a significant loss of function.
Structural Analysis with NMR
To gain deeper insights into the structural changes caused by these mutations, the researchers employed 19F NMR, validated by traditional 13C and 15N techniques. They labeled the FD with fluorinated phenylalanine residues to observe chemical shift perturbations (CSPs). Significant CSPs were observed for K825A, indicating structural changes that enhance its fusogenicity.
Circular Dichroism (CD) Spectroscopy
CD spectroscopy revealed that the K825A mutation increased the α-helicity (a specific secondary structure) of the FD, correlating with its enhanced fusogenicity. Other mutants displayed similar secondary structures to the wild-type FD, suggesting that changes in fusogenicity were not due to global structural changes.
Key Residues and Their Roles
The study's findings emphasize the import
ance of basic residues in the SARS-CoV-2 FD. K825, found in helix one of the FP, and R847, within the FL, were identified as critical for initiating fusion. The K825A mutation, in particular, demonstrated that altering this residue could potentially lead to a more virulent strain of the virus by increasing its ability to fuse with and enter host cells.
Implications for Future Research
This research highlights the potential for specific mutations within the FD to impact the virus's fusogenicity and pathogenicity. Understanding these mechanisms can aid in developing targeted treatments or interventions to block the fusion process, thereby preventing viral entry and infection.
Conclusion
The study conducted by researchers at the University of Maryland sheds light on the critical roles of specific positive residues within the SARS-CoV-2 FD in initiating membrane fusion. These findings enhance our understanding of the molecular mechanisms underlying viral entry and may contribute to developing new strategies to combat COVID-19.
The study's results were published in the peer-reviewed Journal of Biological Chemistry.
https://www.sciencedirect.com/science/article/pii/S0021925824020659
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/israeli-study-reveals-p681-residue-as-key-residue-dictating-cell-fusion-and-syncytia-formation-in-sars-cov-2-variants
https://www.thailandmedical.news/news/covid-19-news-canadian-study-shows-that-quercetin-not-ony-inhibits-sars-cov-2-but-it-also-prevents-syncytium-formation
