Lipid molecules within host cells interact directly with SARS-CoV-2 M protein, controlling its shape and affecting virus assembly
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 06, 2024 5 months, 1 week, 6 days, 13 hours, 58 minutes ago
Medical News: In the world of viruses, the SARS-CoV-2 M protein plays an essential role in assembling infectious virus particles. The M protein, which is the most abundant structural protein in coronaviruses, takes on two forms, or conformations - known as "Mshort" and "Mlong." This dynamic shift between the two shapes is essential for coordinating how the virus builds and releases new infectious particles. However, scientists have long wondered exactly what drives this conformational change and what specific role each state plays in the virus's life cycle.
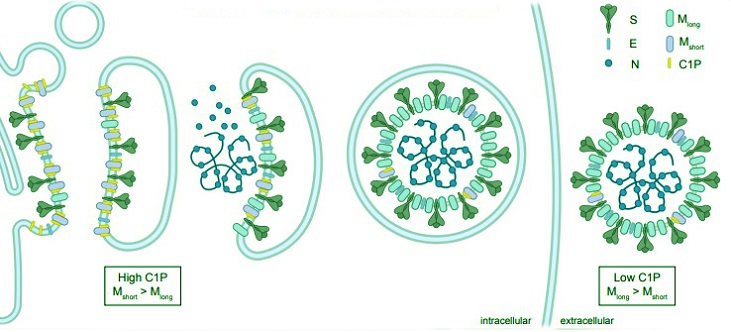 Model for lipid control of M conformational dynamics and coronavirus. assembly.
M protein adopts Mshort and Mlong conformations. Ceramide kinase localization to ERGIC membranes results in high C1P concentrations. C1P binds and stabilizes Mshort in the ERGIC to facilitate recruitment of E and S. Decreasing C1P along the secretory pathway and other potential factors trigger a conformational switch to Mlong that promotes higher membrane curvature and oligomerization necessary for virus assembly and budding.
Model for lipid control of M conformational dynamics and coronavirus. assembly.
M protein adopts Mshort and Mlong conformations. Ceramide kinase localization to ERGIC membranes results in high C1P concentrations. C1P binds and stabilizes Mshort in the ERGIC to facilitate recruitment of E and S. Decreasing C1P along the secretory pathway and other potential factors trigger a conformational switch to Mlong that promotes higher membrane curvature and oligomerization necessary for virus assembly and budding.
This
Medical News report delves into the latest research showing that lipid molecules within host cells interact directly with the M protein, controlling its shape and thus driving virus assembly. The study, conducted by researchers from the University of Chicago, University of California Berkeley, and Purdue University in USA, sheds light on how the M protein’s interaction with specific lipids, especially a lipid called ceramide-1-phosphate (C1P), stabilizes the Mshort conformation, aiding the virus’s assembly process.
Key Findings: Lipid Interactions Control the M Protein's Shape
The researchers discovered that the M protein binds to anionic lipids, including C1P, which is abundant in the cell membranes of host cells where SARS-CoV-2 assembles. Using various molecular and cellular experiments, including molecular dynamics simulations, they found that C1P promotes a transition from the Mlong to the Mshort conformation, essentially holding the M protein in a stable shape that is conducive to virus assembly. This C1P-induced stabilization was observed at a specific binding site on the M protein, as shown through detailed structural studies.
Impact of C1P on Viral Assembly and Structure
Molecular simulations revealed a fascinating insight: the Mlong conformation can transition to Mshort when C1P is present, but without C1P, the Mlong state remains unchanged. This transformation allows the M protein to act as a scaffold, gathering other essential structural proteins in the host cell's endomembrane system. Once the virus assembly reaches a particular stage, C1P levels naturally decrease, encouraging the M protein to switch back to Mlong, promoting virus release and particle budding from the cell.
The stabilization effect of C1P on Mshort creates an ideal environment for the virus
to incorporate spike (S) proteins and envelope (E) proteins into the virus-like particles, which are necessary for the infectious cycle. Through experiments involving cell models, the researchers demonstrated that disrupting the M protein's interaction with C1P led to mislocalization of M within the cell and impaired viral assembly, significantly reducing the virus's ability to infect other cells.
Mechanisms of M Protein Assembly Uncovered
The study team conducted cryo-electron microscopy (cryo-EM) to capture high-resolution images of the M protein in its Mshort form bound to C1P. They identified key residues within the M protein that interact directly with C1P, creating a bridge between the transmembrane and cytoplasmic regions of Mshort. This unique binding site helps explain why the M protein stabilizes in one conformation over the other when C1P is present.
Potential for Antiviral Drug Development
By understanding how C1P binds and stabilizes the M protein, scientists are hopeful that new antiviral drugs targeting these lipid-protein interactions may offer a novel way to prevent virus assembly, thereby blocking infection. One such drug, JNJ-9676, mimics C1P’s binding and stabilization effects on Mshort, effectively trapping the protein in a state that stops virus formation before it can complete its infectious cycle. This insight opens the door to potentially groundbreaking treatments for COVID-19 and related viral infections by targeting virus assembly at the molecular level.
Conclusion
The study provides a compelling view of the M protein's role in SARS-CoV-2 assembly and infection. Through a direct interaction with C1P, the M protein transitions between shapes that determine the virus's ability to gather essential components and release infectious particles. These findings highlight the importance of lipid interactions in viral assembly and could pave the way for new antiviral drugs designed to disrupt these processes. Understanding these molecular mechanics gives researchers a valuable target in the fight against SARS-CoV-2 and potentially other lipid-enveloped viruses.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.11.04.620124v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/vitamin-e-restores-altered-lipid-profiles-in-covid-19-patients-and-reduces-disease-severity
https://www.thailandmedical.news/news/covid-19-news-german-and-swiss-study-finds-that-human-pbmcs-form-lipid-droplets-in-response-to-sars-cov-2-spike-proteins
