Malaysian Researchers Unveil the Role of DPPIV in COVID-19 Cerebrovascular Complications and Post-COVID Fatigue
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 29, 2024 4 months, 2 weeks, 12 hours, 43 minutes ago
Medical News: A team of Malaysian scientists has provided significant insights into the lingering effects of COVID-19, particularly its role in cerebrovascular complications and post-COVID fatigue. Researchers from Universiti Sultan Zainal Abidin, Management and Science University, International Islamic University Malaysia, Universiti Putra Malaysia, Universiti Sains Malaysia, and the International Medical School collaborated on this comprehensive study, revealing the critical role of an enzyme, dipeptidyl peptidase IV (DPPIV), also known as DDP4, in these conditions.
 The Role of DPPIV in COVID-19 Cerebrovascular Complications and Post-COVID Fatigue
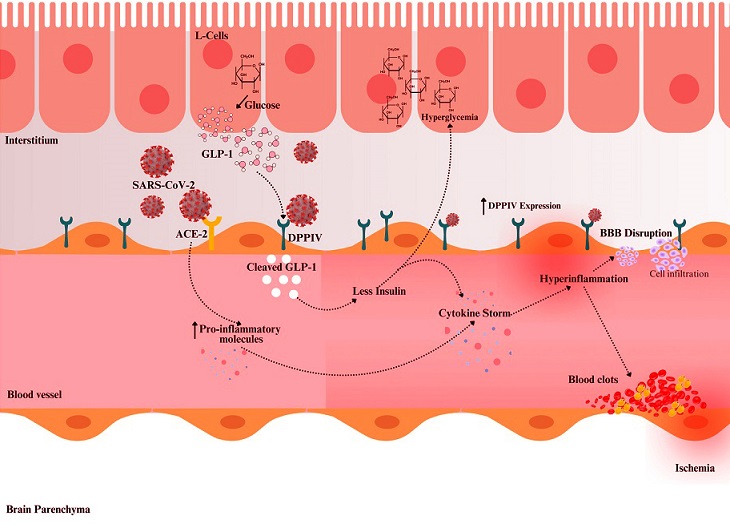
Proposed mechanism illustrating DPPIV-mediated SARS-CoV-2 infection and its impact on glucose metabolism and cerebrovascular health. DPPIV degrades GLP-1 in the bloodstream, leading to reduced insulin secretion and hyperglycemia. This hyperglycemic state, along with the cytokine storm triggered by SARS-CoV-2, exacerbates endothelial dysfunction and inflammation, resulting in blood–brain barrier (BBB) disruption, cell infiltration, blood clot and microthrombus formation, and increased risk of cerebrovascular complications, including stroke. Darker red in the blood vessel structure indicates an inflamed vessel. The upward arrow indicates “increase”.
The Role of DPPIV in COVID-19 Cerebrovascular Complications and Post-COVID Fatigue
Proposed mechanism illustrating DPPIV-mediated SARS-CoV-2 infection and its impact on glucose metabolism and cerebrovascular health. DPPIV degrades GLP-1 in the bloodstream, leading to reduced insulin secretion and hyperglycemia. This hyperglycemic state, along with the cytokine storm triggered by SARS-CoV-2, exacerbates endothelial dysfunction and inflammation, resulting in blood–brain barrier (BBB) disruption, cell infiltration, blood clot and microthrombus formation, and increased risk of cerebrovascular complications, including stroke. Darker red in the blood vessel structure indicates an inflamed vessel. The upward arrow indicates “increase”.
The findings shed light on how DPPIV contributes to neurological and vascular challenges faced by patients recovering from COVID-19. This
Medical News report delves into the study’s findings and its implications for future treatment strategies.
What Is DPPIV, and Why Does It Matter?
Dipeptidyl peptidase IV, or DPPIV, is a versatile enzyme involved in various physiological processes, including glucose metabolism and immune regulation. It has recently been identified as a co-receptor for the SARS-CoV-2 virus, the pathogen responsible for COVID-19. Unlike the more commonly discussed ACE2 receptor, DPPIV not only facilitates viral entry but also amplifies inflammation and metabolic disruptions.
The study highlights how the enzymatic activity of DPPIV exacerbates endothelial dysfunction, promotes the formation of microthrombi (tiny blood clots), and increases cerebrovascular risks such as stroke. By exploring these mechanisms, the researchers emphasize the enzyme’s pivotal role in driving the neurological and systemic complications of post-COVID-19 fatigue.
The Mechanisms Behind Post-COVID Challenges
Post-COVID fatigue and its associated complications are not limited to those who experienced severe illness. Many patients with mild or moderate COVID-19 have reported prolonged symptoms, including brain fog, memory loss, and chronic fatigue. The Malaysian team’s study identified several mechanisms at play, including:
-Endothelial Dysfunction: DPPIV disrupts the function of endot
helial cells, which line the blood vessels, leading to impaired vascular integrity and inflammation.
-Microvascular Damage: The enzyme’s interaction with inflammatory pathways results in the formation of microthrombi, obstructing cerebral blood flow and contributing to cognitive decline.
-Metabolic Dysregulation: By degrading glucagon-like peptide-1 (GLP-1), DPPIV exacerbates hyperglycemia, a significant risk factor for severe COVID-19 outcomes and cerebrovascular damage.
Neurovascular Implications
The research provides evidence of DPPIV’s involvement in brain-related complications, highlighting its role in disrupting the neuro-glia-vascular unit, a critical interface between blood vessels and brain cells. This disruption leads to:
-Blood-Brain Barrier (BBB) Damage: The weakening of the BBB facilitates the infiltration of immune cells into the brain, triggering neuroinflammation.
-Cognitive Impairments: Inflammatory responses mediated by DPPIV contribute to long-term cognitive issues such as memory deficits and difficulty concentrating.
-Increased Stroke Risk: The enzyme’s promotion of clot formation significantly heightens the risk of ischemic stroke in post-COVID patients.
A Potential Therapeutic Target
The study highlights the potential of targeting DPPIV with inhibitors, such as those currently used in diabetes management, to mitigate the long-term effects of COVID-19. These inhibitors could reduce inflammation, prevent microvascular damage, and protect against cerebrovascular complications.
Moreover, the researchers call for further investigation into DPPIV’s role in amplifying the cytokine storm - a hyperactive immune response linked to severe COVID-19. Addressing this pathway could pave the way for novel therapeutic strategies to combat post-COVID fatigue and its neurological sequelae.
Broader Implications for Metabolic and Vascular Health
The findings also underscore the intersection of metabolic disorders and COVID-19 outcomes. Patients with diabetes, hypertension, or obesity - conditions associated with heightened DPPIV activity - face an elevated risk of severe post-COVID complications. This highlights the importance of managing underlying health conditions to mitigate the enzyme’s detrimental effects.
Conclusion: A Step Toward Improved Post-COVID Care
The Malaysian researchers’ study provides a critical piece of the puzzle in understanding the long-term effects of COVID-19. By elucidating the role of DPPIV in cerebrovascular and neurological complications, the study not only enhances our knowledge but also opens the door to targeted therapies. Addressing DPPIV’s enzymatic and inflammatory pathways could revolutionize treatment approaches for post-COVID fatigue and its associated challenges.
The study findings were published in the peer-reviewed journal: Current Issues in Molecular Biology.
https://www.mdpi.com/1467-3045/46/12/811
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-study-alarmingly-shows-omicron-could-be-possibly-evolving-to-shift-its-focus-on-human-receptors-from-ace2-to-ddp-iv,-similar-to-mers
https://www.thailandmedical.news/news/study-reveals-sars-cov-2-binding-to-host-dpp4-receptors-causes-dysregulation-of-proteins-associated-with-diabetes,-leading-to-severe-covid
https://www.thailandmedical.news/news/medical-news-spanish-study-shows-that-covid-19-causes-reduced-efficacy-of-gliptins-in-inhibiting-dipeptidyl-peptidase-4-dpp4-in-diabetic-individuals
https://www.thailandmedical.news/news/thailand-medical-news-exclusive-novel-human-host-receptors-identified-for-sars-cov-2-cell-entry-and-attachment
