Medical Devices Generating Cold Plasma Could Increase The Chances Of Survival For COVID-19 Patients On Ventilators
Source: Thailand Medical Devices Jun 12, 2020 4 years, 10 months, 2 weeks, 2 days, 10 hours, 16 minutes ago
Medical Devices: It is a well-known fact that cold atmospheric plasma (CAP) treatment inactivates various pathogens such as bacteria and viruses.
Medical researchers are now exploring if cold atmospheric plasma CAP is used in the nasal, oral, and pharyngeal cavities, nosocomial super infections in patients with mechanical ventilators, which are a frequent cause of death, could potentially be avoided.
.png)
In the past few weeks, the promising use of cold plasma for treating COVID-19 patients has been discussed with virologists, microbiologists, anesthesiologists, intensive care physicians, and pulmonologists.
Past studies have shown that cold plasmas have a very broad spectrum of efficacy against bacteria including multi-drug resistant organisms such as MRSA and viruses. The latter has been demonstrated on adenoviruses and noroviruses, among others.
https://www.sciencedirect.com/science/article/pii/S0167779920301086 and
https://iopscience.iop.org/article/10.1088/1361-6463/ab1466 and
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6834201/
Hence, the supposition that coronaviruses may also be inactivated by cold plasma seemed obvious. As a result of a collaboration with the von Brunn research group at the Max-von-Pettenkofer Institute in Munich, there are first indications that cold atmospheric plasma can also inactivate coronaviruses in solution. With these promising results, the development of the so-called ‘plasma intensive care’ started at terraplasma medical GmbH , a device for generating gaseous cold plasma, which, in contrast to antiseptic liquids, can also reach angled or hard-to-reach areas in the upper respiratory tract.
The overarching aim is to use the plasma intensive care on mechanically ventilated COVID-19 patients in order to reduce the viral load and to prevent bacterial pneumonia.
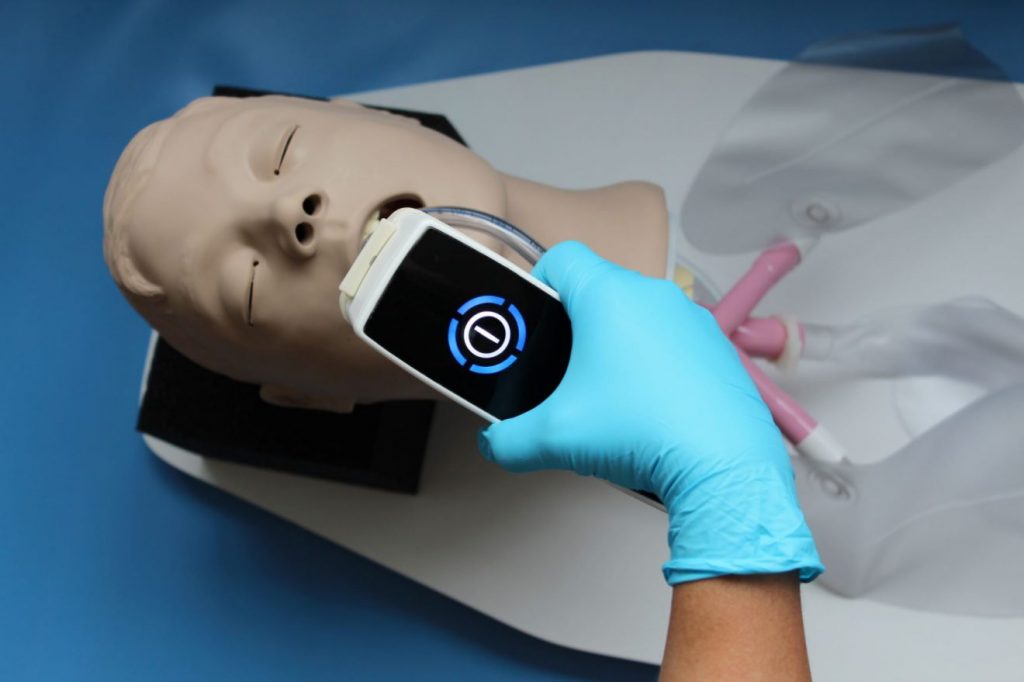
The motivation for this new approach and coming clinical trials is based on an already existing portable medical device called plasma care®, a CE-mark approved medical device that generates cold atmospheric plasma. It was developed for the mobile treatment of acute and chronic wounds. The plasma intensive care is an adaptation of the plasma care® for anti-bacterial and anti-viral treatment of the upper respiratory tract, and is thus intended to improve the oral hygiene of patients with mechanical ventilators in general, and specifically that of COVID-19 patients. The plasma intensive care allows gaseous cold plasma to flow into the nasal, oral and pharyngeal cavities and temporarily floods them. Application of cold plasma is expected to inactivate bacteria and viruses locally to prevent them from penetrating bronchi and lungs. This could reduce the risk of nosocomial pneumonia and increase the chances of survival for these patients.
Typically, cold plasma inactivates bacteria and viruses by mea
ns of several physical and chemical processes. Even in the case of existing antibiotic resistance(s), the effectiveness against bacteria is not impaired. In vitro studies have shown that cold atmospheric plasma achieves a bacterial reduction of up to 99.999 percent on agar within an application time of only 3 minutes.
In addition, it has been demonstrated that various human pathogenic viruses are sensitive towards cold plasma.
The main long-term goal when using cold atmospheric plasma in the nasal, oral, and pharyngeal cavities is to avoid mechanical ventilation by means of preventive treatment of viral infections of the upper respiratory tract. If an early-stage treatment significantly reduces the viral load, it may be possible to avoid the penetration of the virus into the lower respiratory tract. However, as cold plasma can reach the lungs of patients who breathe independently the potential effects resulting therefrom are currently investigated. Research projects are being initiated together with the Fraunhofer Institute for Toxicology and Experimental Medicine and the university hospitals in Regensburg and Munich. terraplasma medical GmbH expects to have the first results in about 6 – 7 months.
For more on the plasmacare device, check out:
https://www.terraplasma-medical.com/en/for-physicians/
For more about the latest
medical devices, keep on logging to Thailand Medical News.
.png)
