Modulations Of Homeostatic ACE2, CD147, GRP78 Pathways In Pulmonary SARS-CoV-2 Infection
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 01, 2024 1 year, 9 months, 3 weeks, 2 days, 7 hours, 41 minutes ago
COVID-19 News: The global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in coronavirus disease 2019 (COVID-19), has brought forth an unprecedented challenge to the healthcare systems worldwide. The pathophysiological consequences of COVID-19 extend beyond respiratory distress, involving a complex interplay of inflammatory responses, vascular dysregulation, and prothrombotic events. Understanding the molecular pathways underlying these intricate interactions is crucial for developing targeted therapeutic interventions. In a new study covered in this
COVID-19 News report, researchers from the New Jersey Medical School at Rutgers University-USA and Boston University School of Medicine-USA delve into the modulations of key cellular receptors - angiotensin-converting enzyme 2 (ACE2), CD147, and glucose-regulated protein 78 (GRP78) - during SARS-CoV-2 infection, exploring their implications on homeostasis, endothelial performance, and the progression of COVID-19.
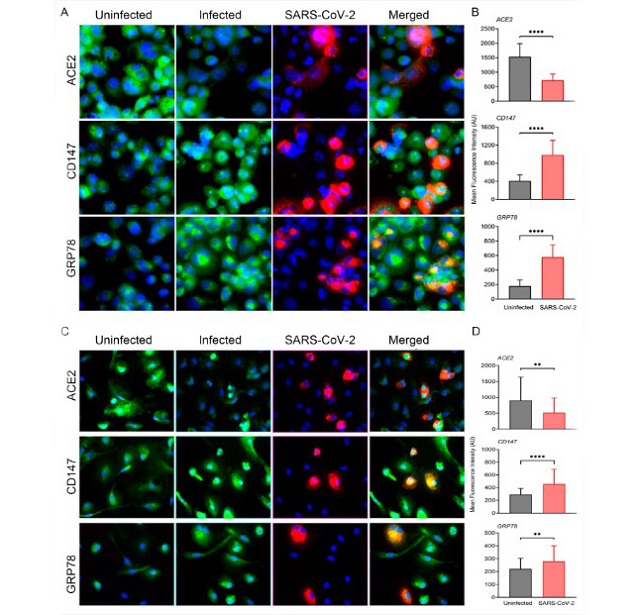 SARS-CoV-2 infection modulates ACE2, CD147 and GRP78 pathways in THP-1/ACE2 and in human MDM/ACE2 macrophages. (A) Representative images of PMA-differentiated THP-1 macrophages transduced with ACE2 with or without SARS-CoV-2 infection (at 5 MOI). The images were taken at 24 hpi with 63× magnification and the scale bar represents 20 μm. Virus (red)- and host cell marker (green)-specific smFISH and nuclear DAPI-staining (blue) are shown. (B) Quantitative measurements of host marker expression from at least 50 cells per field (n = 3 fields per sample with at least 2 samples per condition), normalized against background fluorescence. (C) Representative images of human monocyte-derived macrophages (MDM) transduced with ACE2 and with or without SARS-CoV-2 infection (at 5 MOI). The images were taken at 24 hpi with 63× magnification and the scale bar represents 20 μm. Virus (red)- and host cell marker (green)-specific smFISH and nuclear DAPI-staining (blue) are shown. (D) Quantitative measurements of host marker expression from at least 50 cells per field (n = 3 fields per sample with at least 2 samples per condition), normalized against background fluorescence. Values plotted are mean ± SD (n = 3–4 coverslips per group).
Dissecting the Pathophysiology of COVID-19
SARS-CoV-2 infection modulates ACE2, CD147 and GRP78 pathways in THP-1/ACE2 and in human MDM/ACE2 macrophages. (A) Representative images of PMA-differentiated THP-1 macrophages transduced with ACE2 with or without SARS-CoV-2 infection (at 5 MOI). The images were taken at 24 hpi with 63× magnification and the scale bar represents 20 μm. Virus (red)- and host cell marker (green)-specific smFISH and nuclear DAPI-staining (blue) are shown. (B) Quantitative measurements of host marker expression from at least 50 cells per field (n = 3 fields per sample with at least 2 samples per condition), normalized against background fluorescence. (C) Representative images of human monocyte-derived macrophages (MDM) transduced with ACE2 and with or without SARS-CoV-2 infection (at 5 MOI). The images were taken at 24 hpi with 63× magnification and the scale bar represents 20 μm. Virus (red)- and host cell marker (green)-specific smFISH and nuclear DAPI-staining (blue) are shown. (D) Quantitative measurements of host marker expression from at least 50 cells per field (n = 3 fields per sample with at least 2 samples per condition), normalized against background fluorescence. Values plotted are mean ± SD (n = 3–4 coverslips per group).
Dissecting the Pathophysiology of COVID-19
Moderate to severe cases of COVID-19 are characterized by excessive inflammation in the lungs, driven by an upsurge in proinflammatory cytokines and a loss of immune regulation. This inflammatory milieu contributes to progressive respiratory failure, leading to diffuse alveolar damage and systemic coagulopathy. Thrombosis, capillary inflammation, and endothelial dysfunction become central players, linking alveolar responses to broader vascular disturbances. While the primary targets of SARS-CoV-2 are respiratory organs, the virus's detrimental effects extend to the cardiovascular system, with clinical manifestations of vasculitis observed in multiple organs.
gt;
The Endothelium in Focus
The endothelium, a critical regulator of homeostasis and thrombosis, collaborates with hematopoietic cells expressing key coagulation cascade molecules. Excessive inflammation or injury triggers the endothelium to release regulatory components such as tissue factor (TF) and von Willebrand factor (vWF), promoting platelet aggregation and fibrinolysis control. Activation of endothelium shifts the vascular equilibrium towards a thrombotic state, resulting in endothelial injury and disruption of intercellular junctions, contributing to the hallmark thrombotic complications seen in COVID-19. Despite endothelial and myeloid cells being less prone to productive viral infection, they respond readily to proinflammatory stimuli from infected lung epithelium.
The Trio of ACE2, CD147, and GRP78: Key Players in Homeostasis
Given the crucial role of ACE2, CD147, and GRP78 in maintaining homeostasis and endothelial performance, the researchers hypothesized that their expression modulations are key responses triggered by SARS-CoV-2 infection. ACE2, known as the primary receptor for SARS-CoV-2, is expressed in various lung cell types, including alveolar type II cells, airway epithelial cells, macrophages, and endothelial cells. Previous studies hinted at a positive correlation between ACE2 activation and thrombotic activity. CD147, a multifunctional receptor, plays roles in thrombosis, angiogenesis, and the activation of the urokinase-type plasminogen/plasminogen activator inhibitor-1 (uPA/PAI-1) system. GRP78, a chaperone protein, regulates cellular processes and is involved in thrombosis, endothelial integrity, and anti-apoptotic pathways.
Exploring Modulations in Human Macrophages and Hamster Model
To test their hypothesis, the researchers utilized human macrophages expressing ACE2 in vitro and a Golden Syrian hamster model that recapitulates mild-to-moderate COVID-19 pathophysiology. The results indicated that SARS-CoV-2 infection induced modulations in the expression of ACE2, CD147, and GRP78, correlating with changes in the mRNA expression of coagulation cascade regulators and components of endothelial integrity in infected hamster lungs. Among these markers, tissue factor (TF) emerged as a standout, showcasing the strongest correlation with prothrombotic events during SARS-CoV-2 infection.
Single-Molecule Fluorescence In Situ Hybridization (smFISH): A Window into Infection Dynamics
A noteworthy aspect of the study was the use of the single-molecule fluorescence in situ hybridization (smFISH) method. This technique allowed researchers to determine the peak and resolution phases of SARS-CoV-2 infection, enabling the screening of cellular markers co-expressed with the virus. The findings from smFISH provided valuable insights into the dynamics of infection, supporting the notion that modulations in ACE2, CD147, and GRP78 pathways are detectable in animal model systems, offering a glimpse into the molecular events associated with COVID-19 pathogenesis.
Results: ACE2-Dependent Infection Modulates Homeostasis Pathways
In cultured human macrophages expressing ACE2, researchers observed that SARS-CoV-2 infection led to the downregulation of ACE2-specific RNA, while CD147 and GRP78 RNA levels were upregulated. Interestingly, these modulations were not confined to infected cells but were also observed in non-infected bystander cells, indicating a potential role for virus-associated secreted factors in influencing these changes. Moving from in vitro models to the hamster model, the researchers noted a reduction in body weight, a peak in viral load at 2-4 days post-infection, and a resolution of infection by 12–16 days post-infection. Histological analyses of infected hamster lungs revealed features reminiscent of COVID-19 pathology, including thrombi formation and loss of endothelial cells.
Spatial Dynamics of ACE2, CD147, and GRP78 in Hamster Lungs
To investigate the spatial dynamics of ACE2, CD147, and GRP78 in hamster lungs, smFISH analysis was employed. The results showcased a significant downregulation of ACE2 expression in infected cells at 4 days post-infection, with a partial restoration by 16 days post-infection. Notably, infected cells exhibited a more pronounced downregulation of ACE2 compared to bystander cells. Further analyses using cellular markers such as CD31 and IBA1 highlighted the impact of infection on specific cell populations, reinforcing the intricate relationship between SARS-CoV-2 and the host.
Upregulation of CD147 and GRP78: Implications for Vascular Health
The researchers observed a dramatic upregulation of CD147 and GRP78 RNA in infected hamster lungs, peaking at 4 days post-infection and persisting at elevated levels even after the resolution of infection. The upregulation of these molecules, particularly in virus-harboring cells, indicated their potential involvement in the host's response to infection. CD147, known for its roles in inflammation and thrombosis, and GRP78, with functions in protein processing and signaling, emerged as critical players in the complex cascade of events triggered by SARS-CoV-2.
Prothrombotic Shift and Endothelial Dysfunction: Unveiling Molecular Markers
Examining the prothrombotic shift and endothelial dysfunction associated with COVID-19, the researchers delved deeper into the molecular markers underlying these pathological processes. In addition to the modulations observed in ACE2, CD147, and GRP78, the expression levels of key coagulation cascade regulators were analyzed. Tissue factor (TF), a crucial initiator of the extrinsic coagulation pathway, emerged as a prominent marker, exhibiting a significant upregulation in infected hamster lungs. The dysregulation of TF expression, coupled with alterations in other coagulation factors and endothelial integrity markers, underscored the prothrombotic milieu induced by SARS-CoV-2 infection.
Implications for Therapeutic Interventions
The findings from this study shed light on potential therapeutic targets for mitigating the thrombotic complications associated with COVID-19. Targeting the dysregulated expression of ACE2, CD147, and GRP78 may offer a novel approach to restoring vascular homeostasis and preventing endothelial dysfunction.
Moreover, strategies aimed at modulating the expression or activity of TF and other coagulation cascade components could help alleviate the hypercoagulable state observed in severe COVID-19 cases. By understanding the molecular mechanisms driving thrombosis and endothelial injury, clinicians and researchers can devise more effective therapeutic interventions to improve patient outcomes.
Future Directions: Translating Bench Findings to Bedside Applications
Moving forward, translating the insights gained from preclinical studies to clinical applications represents a critical step in combating COVID-19-related complications. Clinical trials evaluating the efficacy of existing drugs targeting ACE2, CD147, GRP78, and coagulation cascade components are warranted to assess their therapeutic potential in COVID-19 patients. Furthermore, the development of novel therapeutic agents specifically designed to modulate these pathways holds promise for more targeted and efficacious treatments. Collaborative efforts between basic scientists, clinicians, and pharmaceutical companies will be essential for accelerating the translation of bench findings into bedside applications.
Conclusion
In conclusion, the dysregulation of ACE2, CD147, and GRP78 pathways plays a central role in the pathophysiology of COVID-19, contributing to endothelial dysfunction, thrombosis, and vascular injury. Through a combination of in vitro and in vivo studies, researchers have uncovered the dynamic changes in expression levels of these key receptors and their implications for vascular health during SARS-CoV-2 infection. By elucidating the molecular mechanisms underlying COVID-19-related complications, this study provides valuable insights that may inform the development of targeted therapeutic interventions to improve patient outcomes and mitigate the global impact of the pandemic. Continued research efforts aimed at unraveling the intricate interplay between viral infection and host response are crucial for advancing our understanding of COVID-19 pathogenesis and identifying effective strategies for disease management.
The study findings were published in the peer reviewed journal: Cells.
https://www.mdpi.com/2073-4409/13/5/432
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
