Source: Thailand Medical News Nov 04, 2019 6 years, 1 month, 2 weeks, 1 day, 7 hours, 53 minutes ago
A genetic linked disease called Pelizaeus-Merzbacher that leaves neurons without their myelin coating basically has devastating consequences for boys as it’s X-linked. Dr
Nalin Gupta, a professor of neurological surgery and pediatrics at the University of California, San Francisco (UCSF) in a phone interview with
Thailand Medical news commented. “These children have severe developmental delay, so they have inability to walk, inability to talk and perform self-care.Their neurologic function typically does not improve, and usually they actually die during childhood.”
Ten years ago, the biotech firm
StemCells Inc. was looking for a neurosurgeon to try out an intervention that might finally offer some help for these children. Because Gupta had experience conducting surgical clinical trials in kids with disabilities, the company approached him to see if he could transplant neural
stem cells into the brains of boys with Pelizaeus-Merzbacher disease (PMD) an approach that researchers had considered promising for a range of conditions, but which had yet to be proven effective in a clinical trial for any disease.
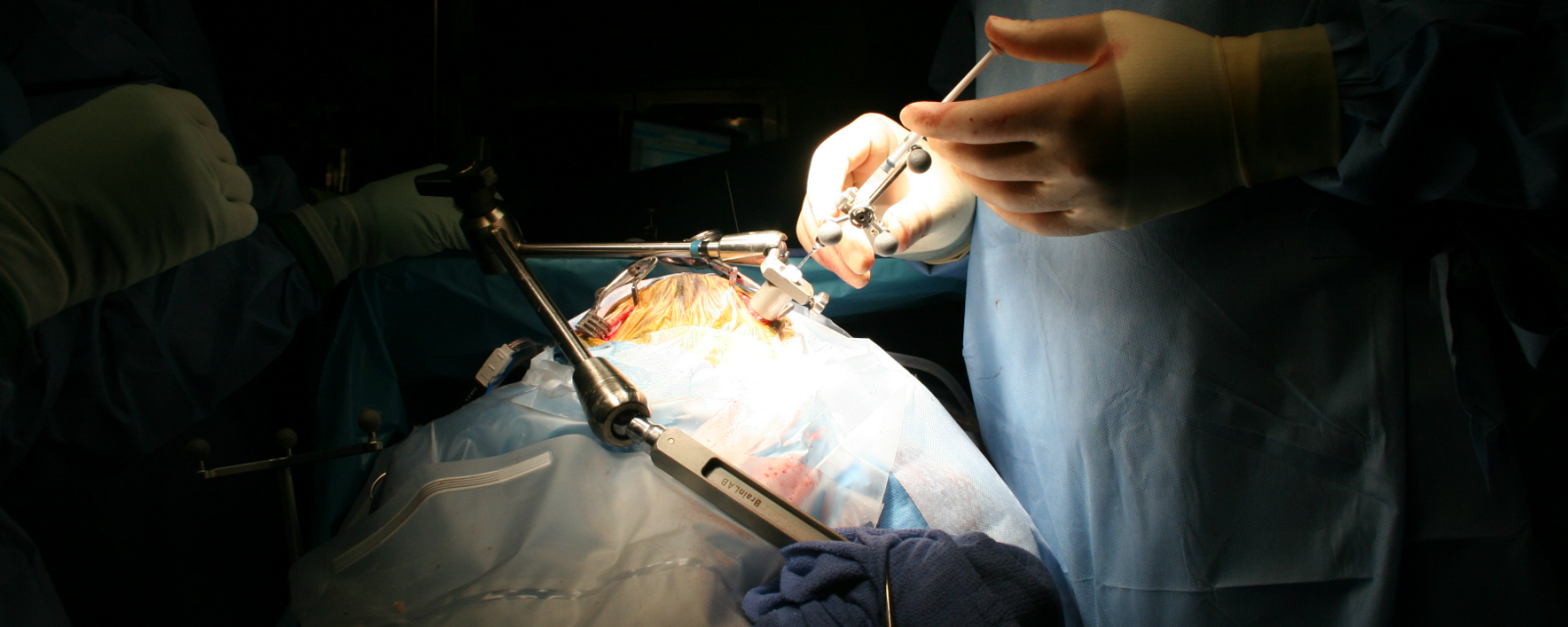 A neurosurgeon injects neural stem cells into the frontal lobe of a child with Pelizaeus-Merzbacher disease who participated in a Phase 1 clinical trial of the transplantation. credit NALIN GUPTA
A neurosurgeon injects neural stem cells into the frontal lobe of a child with Pelizaeus-Merzbacher disease who participated in a Phase 1 clinical trial of the transplantation. credit NALIN GUPTA
Dr Gupta agreed and in 2012, Gupta and colleagues
reported that four boys with PMD who had received pluripotent neural
stem cells in a Phase 1 clinical trial tolerated the procedure, and imaging techniques that indirectly detect myelin indicated they may have had myelination in their brains one year following the transplant. This August 2019, the researchers
reported the results of a long-term follow-up study of those patients all four are still alive at ages 10, 11, 12, and 13. Patients like these who have symptoms of the disease starting at birth typically die in their teens.
Though the medical researchers could not directly examine myelination that would require autopsies, the imaging evidence is promising. There were some clinical improvements, too, although with such a small number of patients and no control group in a trial designed to examine safety, it’s hard to know whether they are attributable to the transplant.
Dr Gupta’s study is the latest report in a series of clinical trials on neural
stem cell transplantation, in which pluripotent neural cell
s taken, in most cases, from the brains of aborted fetuses are expanded in the lab and then injected into the brains or spinal cords of patients with incurable neurological disorders. These include stroke, multiple sclerosis, ALS, spinal injury, and Parkinson’s disease. But for all the effort that has gone in to testing these cells, none have been able to work themselves out of trials and into clinical practice.
When asked which of the human clinical trials have been most successful,
Steven Goldman, a professor of neurology and neuroscience at the University of Rochester, replies, “So far, none of them, right?” To date, no Phase 2 trial to evaluate the efficacy of a neural
stem cell treatment has been completed, he points out. And scientists, Gupta included, are less-than-ecstatic about the methods and outcomes of the clinical trials that have been done so far. Goldman, who was not involved in the PMD experiment, calls it “by far the most rigorous and well controlled.” But that trial can go no further.
Dr Gupta says he and his colleagues “felt that there was sufficient evidence” from the Phase 1 trial to justify a Phase 2. They can’t proceed, however, because
StemCells, the company that funded the research and provided the cells,
closed in 2016. “We don’t actually have a product that we can use even if we wanted to do a Phase 2 study in this disease,” he says.
Medical research on other potential uses for neural
stem cells are also affected by a lack of momentum. “There’s somewhat of a pause in what people are doing in terms of
stem cell therapeutics,” says Gupta. Treatments for conditions such as spinal cord injury and stroke hold the most interest for their potential societal benefit, he says, but the complexity of the changes that occur when the brain or spinal cord are injured meaning regions composed of multiple cell types and networks of connections are just wiped out make for a challenging repair. “We’re probably a long way from being able to transplant a structure that will recapitulate the three-dimensional organization and structure of the brain and spinal cord,” he says. Trials for diseases with more specific defects might be more successful, he adds, such as multiple sclerosis, which like PMD involves demyelination.
Commenting on the issue, Dr
Evan Snyder, the director of the Center for
Stem Cells and Regenerative Medicine at the Sanford Burnam Prebys Medical Discovery Institute in La Jolla, California, there haven’t been enough trials, and certainly not enough under ideal circumstances, to know whether neural
stem cell transplantation can be an effective treatment in humans. “I think the field is too young to know right now if they’re effective. I think the field can just say that they’re safe,” says Snyder. “To be able to know whether
the cells are effective, you really need to be able to put them into an optimal setting where their mechanism of action is optimal, and that kind of trial has never been done yet,” he adds.
The prior research on animal models that laid the foundation for the PMD study, and other studies on diseases involving the loss or absence of myelination, took place in a mouse model called shiverer. These animals have a mutation that prevents their oligodendrocytes from making myelin, such that their neurons are badly insulated and cannot efficiently conduct electrical signals. The shiverer mice have problems with motor functions and self-care along with seizures. They also have a tremor, hence their name.
In late 1999, Snyder’s lab reported in
PNAS that injecting mouse neural
stem cells into the brains of shiverer mice led to the remyelination of neurons as well as some tremor reduction measured by dipping each mouse’s tail in ink and noting the size of the stain it left on a piece of graph paper. Using the model, Goldman’s team later transplanted human glial progenitor cells which are derived from neural
stem cells into shiverer mice, generating chimeras in which mouse neurons became insulated with human myelin. The chimeric mice, as Goldman reported in
Cell Stem Cell in 2008, survived longer and had improved neurological phenotypes, including fewer seizures, compared with untreated controls. In 2012, StemCells Inc., in collaboration with researchers at Oregon Health & Science University and elsewhere, reported in
Science Translational Medicine that transplanting shiverer mice with human neural
stem cells resulted in remyelination in the brain. Also in that issue, Gupta and
StemCells described the one-year results from the PMD trial, which used the same cells for transplantation.
The novel PMD study was not the first trial launched by
StemCells Inc. In 2006, the company launched a Phase 1 trial of neural
stem cell transplantation in children with Batten disease, a fatal condition in which children are missing a lysosomal storage enzyme. “That study was the first study authorized by the FDA for transplantation with neural
stem cells into the brain,” says
Stephen Huhn, a biotech consultant and the former chief medical officer of the company.
The clinical trial, which was completed in 2009, revealed the treatment to be safe, the authors reported in
Journal of Neurosurgery: Pediatrics. Autopies on the brains of several kids who died of the disease during the study suggested that in some patients donor cells had both survived and migrated away from the subcortical and ventricular injection sites and into the basal ganglia, among other locations, Huhn says.
The specimen
stem cells used for this and other
StemCells trials were isolated from the brain of a single aborted fetus, expanded as balls of cells called neurospheres, and frozen for later use. Before injection into patients, the cells were thawed, cultured for two weeks, and dissociated, so that what was injected was no longer a neurosphere but a cluster of cells, according to Huhn. Because the neural
stem cells were donor-derived, patients were given immunosuppressant drugs for several months following the transplant to prevent rejection.
Utilizing the same procedure and stock of cells, Gupta and colleagues transplanted neural
stem cells into the brains of the four boys with PMD in a Phase 1 trial that began in 2009 and ran through 2012,the same trial whose long-term follow-up results came out this summer. One year after transplantation, diffusion tensor imaging, an MRI-based technique that lets researchers indirectly observe myelinated axons of the boys’ brains suggested that myelination had occurred.
From early 2012 to 2015, the company ran a Phase 1/2 trial of neural
stem cell transplantation for age-related macular degeneration. The treatment proved safe, and there was also evidence of a treatment effect a slowing of the retinal damage called “geographic atrophy” and improvements in visual function in some patients, says Huhn.
Simultaneously , the firm was engaged in a Phase 1/2 trial of
stem cell transplantation for patients with injuries to the thoracic region of the spine. The treatment proved safe, and Huhn notes that several participants seemed to have “sensory improvement below the level of injury, which would imply that the stem cells were having a treatment effect.”
Unfortunately company’s run of auspicious results did not last forever: Its Phase 2 trial of neural
stem cells to treat cervical spinal cord injury, which began in 2014, terminated two years later after an independent review of the emerging data found that the study was unlikely to show a statistically significant treatment effect, Huhn says. For that same reason, a follow-up study on the same patients also ended in 2016, he adds.
Around that time,
StemCells Inc. shut down.
STAT reported that the reason was disappointing results from the spinal cord study.The company’s work was not in vain, says Huhn, as it demonstrated that the approach is safe and might be worth pursuing. These are challenging disorders, Huhn says, adding that “the fact that we saw even glimmers of an effect was for us very promising that cellular therapy could well have a place in the treatment of some neurological disorders.”
Dr Snyder, who was not involved in the work, said that the PMD trial suffered from the limitations of the clinical trials system. “The unfortunate thing is the way clinical trials are designed, you only get a patient who has failed every other intervention, is very deep into the disease, and almost has no chance of anything changing the course,” he says. This problem is not unique to the PMD trial but applies to all neural
stem cell clinical trials to date, Snyder says. In the Batten disease trial, for instance, the patients had little hope of recovery, Snyder notes. Three of the six participants had died of their disease by the time the researchers stopped collecting data. “Where
stem cells are going to be most useful, ultimately, is going to be the early stages of a disease where there are regions that can be rescued, and where the cells are placed in a position where they can distribute themselves throughout the region that needs to be fixed. And no clinical trial has ever met those criteria.”
Though the follow-up PMD study revealed some myelination, there was not a lot of it, notes Goldman, who was not involved in the work. “There’s some evidence for local remyelination around the region of the transplants, but there was nothing that was dispersed or broad, and these patients need really widespread remyelination,” he says. He believes that there was not more widespread and robust myelination in the PMD patients because of the cell type used. While neural
stem cells can give rise to oligodendrocytes, astrocytes, and neurons, they not very efficient at making oligodendrocytes, he says. And, he adds, they do not migrate much, which is necessary for them to have widespread effects. In contrast, human glial progenitor cells, which are produced from neural
stem cells and give rise to both oligodendrocytes and astrocytes, are more migratory, says Goldman, and for this reason, the field has shifted away from neural
stem cells and toward glial progenitor cells for transplantation. Goldman has
trials of his own in the works using a neural
stem cell derivative to treat multiple sclerosis and PMD through a company he cofounded, Oscine Therapeutics.
Other new trials are currently underway. Researchers at Emory University and the University of Michigan, with funding from the company Neuralstem, have completed a
Phase 1 study of neural stem cells to treat ALS and, according to ClinicalTrials.gov, a Phase 2 clinical trial is ongoing. There’s a Phase 2/3 trial of nasally delivered neural
stem cells to treat Parkinson’s disease
enrolling in China. And there’s an active
Phase 1 trial for Parkinson’s disease in Australia using human parthenogenetic neural
stem cells derived from unfertilized eggs, rather than fetal tissue.
Early 2019, Snyder received a
California Institute for Regenerative Medicine (CIRM) grant to do work leading up to cell-based therapies for babies who are at risk for developing cerebral palsy due to perinatal asphyxia, or oxygen and blood deprivation in the womb, he says. Within the first few days of life, the researchers plan to do brain imaging to identify babies with regions of the brain where cells are injured but not dead, he says, then transplant neural
stem cells. “The injury’s still very fresh and cells are sort of teetering on a knife edge. They can either go on to die or they can go on to live, and the transplanted
stem cells make factors that push them in the direction to live,” Snyder says. “If that happens, the prediction is the babies will do much better.”
There’s only a short window, when cells are damaged but not dead, during which a neural
stem cell transplant can work, he adds. Other trials in older patients with more advanced disease, he suggests, may have missed their optimal treatment windows. Snyder predicts that if the right patients are transplanted with the right neural
stem cells at the right time, “I think then, under those circumstances, now you’re going to start seeing not just safety but real efficacy.
Many medical professionals believe that neural
stem cells therapies hold hope and possible cure for a variety of diseases but just need proper clinical trials to proceed and regulatory measures to be handled.
