Neutrophil Activity And Lung Tissue Destruction In Fatal COVID-19 Cases And Long COVID
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 27, 2024 1 year, 10 months, 1 day, 13 hours, 43 minutes ago
COVID-19 News: The COVID-19 pandemic has left an indelible mark on global health, revealing the devastating consequences of respiratory viral infections. While many infected individuals recover with mild symptoms, severe cases can lead to respiratory failure, with fatal outcomes in approximately 1% of patients.
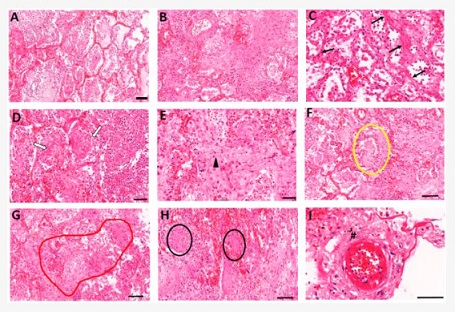 Lung histopathology analyses of COVID-19 patients. Autopsy lung samples collected from COVID-19 patients show major patterns of (A) widespread diffuse alveolar damage and (B) fibrosing organizing pneumonia. Fibrotic changes in COVID-19 patients show variable patterns including (C) interstitial fibrotic development (black arrows), (D) intra-alveolar fibrin deposition and organizing fibrosis, showing spreading of fibrosis in alveoli (white arrows), and (E) diffuse alveolar fibrosis within small airways (arrowhead). (F) Loose myxoid fibroblastic proliferation extended between the alveoli-forming Mason bodies in alveolar and bronchiolar regions (yellowoval). (G) Bronchiolitis obliterans organizing pneumonia (BOOP)-like areas (redoutline). (H) Squamous cells forming distinct nodules (blackovals). (I) Disrupted small blood vessels also displayed perivascular fibroblast proliferations (hash mark). Scale bars = 100 μm.
Lung histopathology analyses of COVID-19 patients. Autopsy lung samples collected from COVID-19 patients show major patterns of (A) widespread diffuse alveolar damage and (B) fibrosing organizing pneumonia. Fibrotic changes in COVID-19 patients show variable patterns including (C) interstitial fibrotic development (black arrows), (D) intra-alveolar fibrin deposition and organizing fibrosis, showing spreading of fibrosis in alveoli (white arrows), and (E) diffuse alveolar fibrosis within small airways (arrowhead). (F) Loose myxoid fibroblastic proliferation extended between the alveoli-forming Mason bodies in alveolar and bronchiolar regions (yellowoval). (G) Bronchiolitis obliterans organizing pneumonia (BOOP)-like areas (redoutline). (H) Squamous cells forming distinct nodules (blackovals). (I) Disrupted small blood vessels also displayed perivascular fibroblast proliferations (hash mark). Scale bars = 100 μm.
Understanding the pathogenesis of fatal COVID-19 is crucial for devising effective therapeutic strategies and unraveling the mysteries of long COVID, a condition characterized by persistent symptoms even after recovery. In this comprehensive study covered in this
COVID-19 News report, researchers from the University of Tennessee Health Science Center, Adichunchanagiri University, University of Pennsylvania, Danylo Halytsky Lviv National Medical University, Pulmonology Lviv Regional Diagnostic Center, and University of Memphis delve into the intricate details of lung pathology in fatal COVID-19 cases.
Exploring Pulmonary Fibrosis and Alveolar Epithelial Architecture
Pulmonary fibrosis, severe alveolitis, and the inability to restore alveolar epithelial architecture are identified as primary causes of respiratory failure in fatal COVID-19 cases. While these phenomena have been observed, the factors contributing to abnormal fibrosis in critically ill COVID-19 patients have remained elusive. This study aims to bridge this knowledge gap by conducting a detailed analysis of the histopathology of lung specimens obtained from eight COVID-19 and six non-COVID-19 postmortems.
Unraveling Extracellular Matrix (ECM) Changes
The researchers focused on assessing the distribution and changes in extracellular matrix (ECM) proteins, specifically elastin and collagen, in the lung alveoli through morphometric analyses. Elastin and collagen are major structural components of the alveoli, providing essential mechanical support and elasticity. The findings of this study reveal a significant degradation of elastin fibers along the thin alveolar walls of the lung parenchyma. Importantly, this pr
ocess precedes the onset of interstitial collagen deposition and widespread intra-alveolar fibrosis.
Connecting Neutrophils to Elastin Degradation
The study sheds light on the critical role of neutrophils and neutrophil enzymes, particularly neutrophil elastase (NE), in the pathogenesis of COVID-19. The loss of elastin is found to be strongly correlated with the induction of NE, a potent protease known for degrading ECM. Immunoblotting of lung autopsy tissue extracts confirms the degradation of elastin. Increased staining for peptidyl arginine deiminase, a marker for neutrophil extracellular trap (NET) release, and myeloperoxidase, an enzyme generating reactive oxygen radicals, further indicate active neutrophil involvement in lung pathology.
Implications for COVID-19 Complications
This study not only highlights the immediate implications for fatal COVID-19 cases but also extends its relevance to severe COVID-19 complications, including long COVID, and other chronic inflammatory and fibrotic disorders. The authors emphasize the importance of understanding the role of neutrophils and elastin degradation in impaired alveolar function, arguing that elastolysis and alveolitis trigger abnormal ECM repair and fibrosis.
Detailed Analysis of Study Results
The researchers examined lung autopsy samples from eight fatal COVID-19 cases and six non-COVID-19 lungs. The subjects had a mean age of 60.3 years, with seven out of eight COVID-19 patients hospitalized for more than 10 days. The study observed histopathological manifestations, including diffuse alveolar damage (DAD), fibrosing organizing pneumonia, and the presence of fibrotic changes in all COVID-19 patients. The onset of fibrotic changes appeared focally in the alveolar interstitium, causing disorganization of alveolar architecture.
Elastin Degradation and Collagen Accumulation
The most notable result of the study was the extensive degradation of elastin in the extracellular matrix of COVID-19 lungs. Elastin fibers displayed disintegration, particularly along the walls of alveoli with extensive necrosis of the alveolar epithelium. Quantitative measurements confirmed significantly reduced lengths of elastin fibers in COVID-19 lungs. The study also identified increased collagen deposition in the alveolar interstitium of COVID-19 patients, with a four-fold increase in the collagen/elastin ratio compared to control lungs.
Neutrophil Aggregates and NETosis
The study revealed massive neutrophil aggregates within airways and alveolar spaces of COVID-19 lungs, displaying strong immunostaining for granule proteins, including NE, myeloperoxidase, and PAD4. Increased levels of total citrullines in COVID-19 lungs indicated active NETosis. The analysis of plasma samples from hospitalized COVID-19 patients showed a significant increase in NE-A1AT complexes, suggesting elevated extracellular release of NE.
Discussion and Implications
The findings of this study provide crucial insights into the pathologic manifestations of fatal COVID-19, emphasizing the central role of elastolytic activity in exacerbating pulmonary pathology. Dysregulated neutrophil activity and NETosis contribute to elastin degradation, ECM remodeling, and fibrotic changes. The study highlights the potential of NE-A1AT complexes as biomarkers for diagnosing neutrophil activation and tissue damage in COVID-19 patients.
Future Directions and Therapeutic Implications
The researchers suggest that a better understanding of the mechanisms of elastin degradation could lead to the identification of novel therapeutic targets to prevent pulmonary fibrosis in COVID-19 patients. The study also has broader implications for patients with long COVID-associated lung impairment, emphasizing the need to address fibrotic scarring that drastically reduces lung function. Ongoing efforts to reduce or ameliorate the excessive activity of neutrophils are crucial for mitigating the debilitating effects of long COVID.
Conclusion
In conclusion, this comprehensive study provides a detailed exploration of the intricate connections between neutrophil activity, elastin degradation, and the development of pulmonary fibrosis in fatal COVID-19 cases. The findings not only contribute to our understanding of the immediate pathology but also offer valuable insights into potential therapeutic targets for preventing long-term complications associated with COVID-19. As the world continues to grapple with the aftermath of the pandemic, ongoing research in this area will be crucial for improving patient outcomes and informing future strategies for managing respiratory viral infections.
The study findings were published in the peer reviewed journal: Biomolecules.
https://www.mdpi.com/2218-273X/14/2/236
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
