Source: Thailand Medical News Jul 31, 2019 6 years, 5 months, 2 weeks, 5 days, 8 hours, 49 minutes ago
Researchers from the University of Arizona Cancer Center have developed a new and more effective treatment protocol for the treatment of advanced biliary tract cancers using a combination of drugs. This new treatment extends the survival rates of patients and also improves the quality of life compared to current conventional treatments in which such patients normally have survival rates of less than a year upon the disease being discovered of being at this stage.
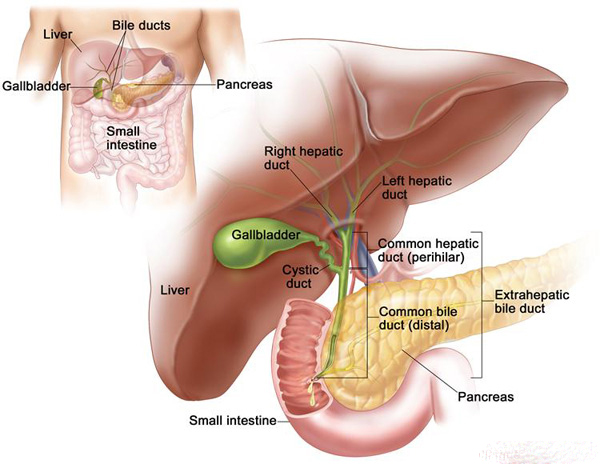
Biliary tract (liver, gallbladder and pancreas) cancers are a growing phenomenon in Thailand, Cambodia, Vietnam and Myanmar due to dietary habits, especially from the rural areas. In the US Biliary tract cancer is a rare with only about 15,000 cases per year.
The standard treatment since 2012 for biliary cancer has been a chemotherapy regimen involving the combination of drugs cisplatin and gemcitabine.
The research team instead studied the effects of using a third drug to the combination, nab-paclitaxel, which had already been approved by the US FDA earlier for pancreatic cancer and other cancers. The researchers considered its potential to aid in the treatment of biliary tract cancers because of their similarities to pancreatic cancer due to a dense stroma, or protective barrier, which surrounds the tumor.
“Chemo cannot access the tumor because of this protective stromal barrier, Nab-paclitaxel in pancreatic cancer is thought to dissolve away the stroma, and enhances chemotherapy delivery to the tumor. So my thought was that if this works in pancreatic cancer, and there is an equally important role of the stroma in biliary cancers, hence we should explore adding nab-paclitaxel to the combination regimen.” commented Dr. Rachna Shroff , lead researcher in an exclusive interview with Thailand Medical News.
In the phase ii “single arm” clinical trial that included sixty patients receiving the drug combo involving the three drugs that was conducted at the University of Texas M.D. Anderson Cancer Center and the Mayo Clinic Arizona, the findinsg showed that gemcitabine-cisplatin plus nab-paclitaxel prolonged progression-free survival and overall survival, compared to historical data for the standard treatment.
Past data showed that the standard median progression-free survival of gemcitabine-cisplatin was eight months and overall survival was 11.2 months.However by adding nab-paclitaxel to the regimen, the study found progression-free survival increased to 11.8 months and overall survival extended to 19.2 months. The response rate to the three-drug treatment improved to 45%, compared with 26% with gemcitabine-cisplatin.
Another positive finding was that 12 of the 60 patients had a dramatic response to the therapy and were converted from palliative treatment, designed to relieve symptoms, to potentially curative treatment with a resectable tumor,one that surgically could be removed. Among those, two had a pathologic complete response, meaning all of the tumor was dead when removed.
A phase III study through the Southwest Oncology Group, or SWOG 1815, began last December 2018 with an estimated completion set for Jan. 1, 2024. Shroff is the principal investigator for the study, which is the first randomized phase III clinical trial in biliary cancers in the United States.
The researchers hope that gemcitabine-cisplatin plus nab-paclitaxel will become a new standard treatment for all newly diagnosed patients with
biliary cancer based on the oncome of this new trial.
Reference: Rachna T. Shroff et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers, JAMA Oncology (2019). DOI: 10.1001/jamaoncol.2019.0270
