New immunotherapy protocol for Multiple Sclerosis (MS).
University Of Queensland Nov 21, 2018 7 years, 1 month, 5 days, 10 hours, 2 minutes ago
A world-first clinical trial of a new cellular immunotherapy for multiple sclerosis (MS) has improved symptoms and quality of life for the majority of patients.
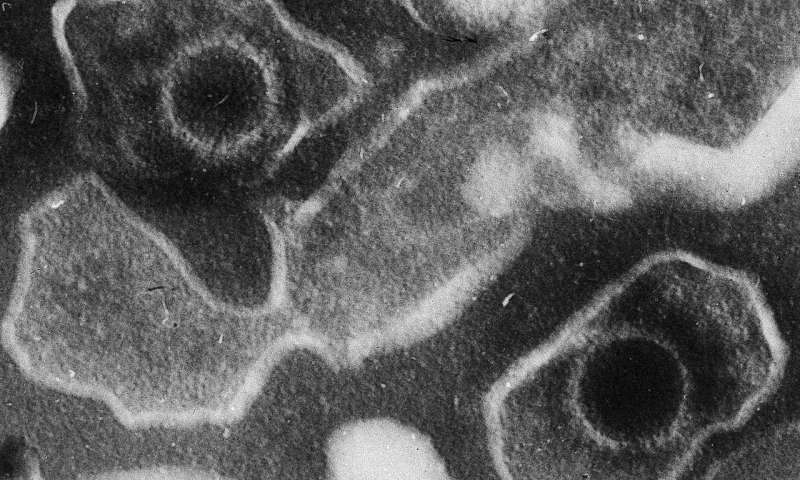
This electron microscopic image of two Epstein Barr Virus virions (viral particles) shows round capsids—protein-encased genetic material—loosely surrounded by the membrane envelope. Credit: DOI: 10.1371/journal.pbio.0030430.g001
The treatment targets the Epstein-Barr virus (EBV), and is based on a theory formulated by University of Queensland and Royal Brisbane and Women's Hospital (RBWH) researcher Professor Michael Pender.
In 2003, Professor Pender proposed that MS resulted from an accumulation of EBV-infected cells in the brain, and that a therapy targeting EBV could stop the progression of the disease.
The cellular immunotherapy was developed by Professor Rajiv Khanna and his team at QIMR Berghofer Medical Research Institute, and the phase I clinical trial was conducted in collaboration with Professor Pender and colleagues.
Professor Pender said 10 patients – five with secondary progressive MS and five with primary progressive MS – received four doses of the cellular immunotherapy treatment at the RBWH.
"Seven of these patients showed improvements," he said."Without this treatment, we would have expected their symptoms to continue to get worse."Improvements ranged from reduced fatigue and improved productivity and quality of life to improvements in vision and mobility."Importantly, we found the treatment was safe and without serious side-effects."Our findings add to the mounting evidence that EBV infection plays a role in the development of MS."
Professor Khanna said it was the first time a T cell immunotherapy had been used to treat an autoimmune disease.
"We have already used these cellular immunotherapies to treat
different types of cancer and viral infections," he said.
"This clinical trial is a breakthrough because, for the first time, we have found these treatments are safe and have had positive improvements in an autoimmune disease.
"This trial opens the door to develop similar cellular immunotherapies for certain other autoimmune conditions.
"From this phase I trial, we have also discovered what cell properties produce the best results for the patients.
"We can now apply this knowledge to cellular immunotherapies for other diseases to try to ensure the best results for all patients."
The phase I clinical trial started in November 2015. A phase II trial, sponsored by Atara Biotherapeutics, is planned for several locations in Australia and the United States.
Multiple Sclerosis, which is a condition of the central nervous system, is estimated to affect more than 25,000 Australians, with symptoms including problems with coordination, impaired balance, weakness of the limbs, cognitive problems and memory loss.&
lt;br />
Most MS patients are diagnosed with a relapsing remitting form of the disease, but some go on to develop a secondary progressive form in which disability gradually worsens.
A small proportion is diagnosed with a primary progressive form from the outset. A range of treatments is available to prevent attacks in relapsing remitting MS, but there are limited treatment options for people with progressive forms of MS.
Professor Khanna said cellular immunotherapy involved taking blood from patients, extracting T (immune) cells, and "training" them in the laboratory to recognise and destroy EBV in the brain lesions of MS patients .
The cellular immunotherapies were manufactured at QIMR Berghofer's Q-Gen Cell Therapeutics, one of the largest cell therapy manufacturing facilities in Australia.
The trial was funded by MS Queensland, MS Research Australia, Perpetual Trustee Company Ltd and private donations.
The results of the clinical trial have been published in
JCI Insight.
Reference: Michael P. Pender et al. Epstein-Barr virus–specific T cell therapy for progressive multiple sclerosis, JCI Insight (2018). DOI: 10.1172/jci.insight.124714