New insights from stem cell research reveals role of inflammation and macrophages in atrial fibrillation
Chitra Varughese Fact checked by:Thailand Medical News Team Sep 10, 2024 1 year, 3 months, 3 weeks, 1 day, 12 hours, 28 minutes ago
Medical News: Atrial fibrillation (AF) is one of the most common types of heart arrhythmias, affecting an estimated 1.5–2% of the population globally. As the world ages, these numbers are expected to rise, with an increased burden on healthcare systems. Current treatments like anti-arrhythmic drugs and ablation procedures provide some relief, but often require repeated interventions, leaving a significant portion of patients without a permanent solution. Inflammation is increasingly seen as a key player in AF, but the exact mechanisms through which it triggers and sustains this condition are still under investigation. This
Medical News report highlights groundbreaking research that explores the direct role of inflammation and macrophages in inducing AF-like conditions using advanced stem cell technologies.
 New insights from stem cell research reveals role of inflammation and macrophages in atrial fibrillation
Inflammation: The Hidden Driver of AF
New insights from stem cell research reveals role of inflammation and macrophages in atrial fibrillation
Inflammation: The Hidden Driver of AF
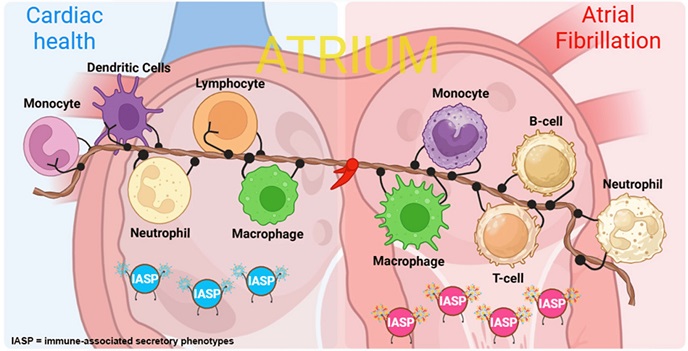
In the past few years, scientists have noted the high levels of inflammation markers in the blood of patients with AF, along with the presence of pro-inflammatory macrophages in atrial tissue. While these connections have been established, the question remains whether inflammation is a cause or consequence of AF.
A new study from an international group of researchers aimed to answer this very question by creating an innovative coculture model using human-induced pluripotent stem cells (hiPSCs). This allowed them to model the inflammatory process in human heart cells and directly observe its effects on atrial fibrillation-like conditions. The researchers are from institutions such as Ncardia Services B.V. (Netherlands), Cardiovascular Research Institute Maastricht (Netherlands), Instituto de Investigación Sanitaria La Fe (Spain), and the University Heart Center Freiburg (Germany).
The scientists focused on M1 macrophages, a subtype known for its role in inflammation. In their model, these activated M1 macrophages were combined with human atrial cardiomyocytes derived from hiPSCs to mimic the inflammatory environment in the heart. Electrophysiological analysis showed that these M1 macrophages caused irregular beating patterns and reduced the electrical activity of the cardiomyocytes - hallmarks of AF. The study dives deeper into how these findings pave the way for future treatments.
A Closer Look at the Study
To explore the relationship between inflammation and AF, the research team took two lines of hiPSCs from healthy individuals and differentiated them into atrial cardiomyocytes and M1 macrophages. These cells were then placed in a coculture system designed to simulate AF-like conditions without any external electrical pacing. Instead, the researchers relied solely on the immune-induced stress to trigger arrhythmic activity.
The team found that M1 macrophages caused severe beat irregularities in the cardiomyocytes. When these cells were examined more closely using multi-electrode array recordings, a significant reduction in electrical signals (known as electrogram amplitude
) was observed. The speed and coordination of electrical signals, known as conduction velocity and homogeneity, were also disrupted. This provided strong evidence that inflammation is indeed a direct cause of arrhythmia.
The study further explored the gene expression changes that occurred in the cardiomyocytes exposed to M1 macrophages. Genes critical for heart function, such as SCN5A, KCNA5, ATP1A1, and GJA5, were significantly downregulated. These genes are essential for maintaining proper ion flow and electrical activity in the heart. Notably, the changes in gene expression closely resembled those observed in clinical cases of AF, providing further support for the study’s model.
Reversing the Effects of Inflammation
One of the most exciting aspects of the study was its exploration of potential treatments for inflammation-induced AF. The research team tested glucocorticoids - commonly used anti-inflammatory drugs - on their coculture model. The drugs included hydrocortisone and dexamethasone, both of which were found to reverse the damaging effects caused by M1 macrophages.
Specifically, glucocorticoids restored the electrogram amplitude and improved the conduction velocity, helping the cells regain their normal electrical patterns.
Importantly, the drugs significantly reduced the irregularity in the beating of the cardiomyocytes by 46% for hydrocortisone and 21% for dexamethasone.
However, non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen did not produce the same beneficial effects, highlighting the specific role of glucocorticoids in combating inflammation-related arrhythmia.
In addition to restoring electrical function, glucocorticoids also reversed many of the gene expression changes observed in the cardiomyocytes. Genes involved in the inflammatory response, such as CXCL8 (a chemokine), were downregulated, while those responsible for electrical function were restored. These findings suggest that targeting the inflammatory response in AF patients could be a promising new therapeutic avenue.
Key Findings and Implications
This study provides the first direct evidence that inflammation, driven by M1 macrophages, can cause AF-like conditions in human heart cells. By using an innovative coculture model, the research team was able to mimic the conditions found in AF patients and observe the direct impact of inflammation on heart function. The successful reversal of these effects through glucocorticoids offers hope for developing new treatments that specifically target inflammation in AF patients.
This new understanding of how inflammation affects the heart opens the door to more personalized treatments. The potential to intervene at the level of immune cells and inflammation could revolutionize how we approach AF treatment. In particular, it highlights the need for more research into the immune system's role in heart disease and the potential for anti-inflammatory drugs to play a larger role in treatment protocols.
Conclusion
The findings from this study shed new light on the complex relationship between inflammation and atrial fibrillation. By demonstrating that M1 macrophages can directly induce arrhythmia-like conditions, the research offers new insights into the underlying causes of AF and paves the way for future therapies. With inflammation now identified as a potential target, doctors may soon have new tools to prevent and treat AF in its earliest stages.
The study findings were published in the peer-reviewed journal: Stem Cell Research & Therapy.
https://link.springer.com/article/10.1186/s13287-024-03814-0
For the latest on atrial fibrillation, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/a-deep-dive-into-atrial-fibrillation-management-and-the-usage-of-amiodarone-in-septic-shock-patients
https://www.thailandmedical.news/news/new-study-reveals-effective-treatment-for-recent-onset-atrial-fibrillation
