Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 18, 2025 8 months, 4 weeks, 3 hours, 58 minutes ago
Medical News: A groundbreaking study led by researchers from the All India Institute of Medical Sciences (AIIMS), Indian Institute of Technology (IIT) Kharagpur, and several other prestigious institutions has unveiled critical insights into the activity of NSP12, a key protein in the replication machinery of SARS-CoV-2. This
Medical News report delves into the vital role of NSP12 and how its interactions with NSP7 and NSP8 regulate its function, offering potential avenues for antiviral drug development. NSP12, a central component of the viral RNA-dependent RNA polymerase (RdRp) complex, is instrumental in the replication and transcription of the SARS-CoV-2 genome.
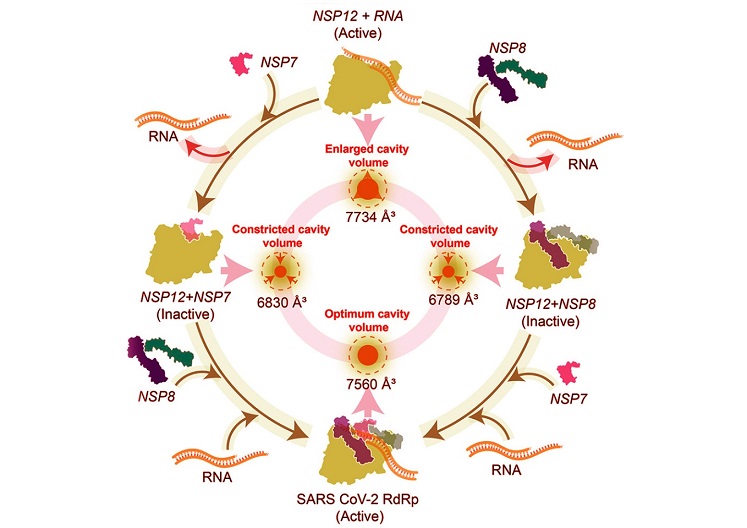 Graphical Abstract - New Insights into SARS-CoV-2 NSP12 Activity
Unpacking the Study’s Key Findings
Graphical Abstract - New Insights into SARS-CoV-2 NSP12 Activity
Unpacking the Study’s Key Findings
Researchers meticulously reconstituted the RdRp holoenzyme using recombinant proteins NSP12, NSP7, and NSP8. The study revealed that while NSP12 alone exhibits notable polymerase activity, its function is significantly enhanced when paired with both cofactors. Interestingly, the presence of only one cofactor - either NSP7 or NSP8 - led to a complete inhibition of NSP12’s activity and caused the detachment of RNA templates.
Methodology Highlights
The researchers employed advanced molecular biology techniques to study the structural and functional dynamics of the NSP12-RNA complex. Key steps included:
-Protein Expression and Purification: Recombinant NSP12, NSP7, and NSP8 proteins were expressed in bacterial systems and purified for in vitro experiments.
-Reconstitution of RdRp Complex: The functional holoenzyme was reassembled by mixing the proteins in specific ratios, mimicking natural viral conditions.
-Molecular Dynamic Simulations: Using computational models, the team analyzed changes in NSP12’s structure when interacting with its cofactors.
Biochemical and computational analyses offered critical insights:
-NSP12 Alone: The protein demonstrated independent polymerase activity, albeit less efficient compared to the fully assembled RdRp holoenzyme.
-NSP7 and NSP8 Roles: When present individually, these cofactors constricted the RNA entry channel of NSP12, disrupting its ability to bind RNA and perform replication effectively. This constriction was particularly pronounced with NSP8.
-Synergistic Functionality: Together, NSP7 and NSP8 not only stabilized NSP12 but also optimized the RNA entry channel, enabling efficient replication.
Implications for Drug Development
The findings suggest that NSP7 and NSP8 serve dual roles as enhancers and inhibitors of NSP12 activity. The study’s molecular dynamic simulations highlighted significant structural changes in NSP12 when bound to e
ither cofactor alone. These alterations constricted the RNA entry channel, rendering it unsuitable for RNA binding.
However, when both cofactors were present, the RNA entry tunnel remained accessible and functional. This dynamic interplay underscores the potential of targeting NSP12’s interactions with NSP7 and NSP8 as a strategy for antiviral therapy. Inhibiting these interactions could disrupt the formation of the replication-transcription complex (RTC), halting viral replication.
Broader Context and Impact
SARS-CoV-2, the virus responsible for COVID-19, has caused immense global health challenges. NSP12’s central role in the virus’s replication makes it a prime target for therapeutic interventions. While drugs like Remdesivir and Molnupiravir target NSP12’s polymerase activity, their efficacy is limited by factors such as drug resistance and viral mutations. This study’s insights into the regulatory roles of NSP7 and NSP8 provide a fresh perspective on potential drug targets, especially for designing inhibitors that prevent the formation of functional RdRp complexes.
Conclusions
The study reaffirms NSP12’s critical role in SARS-CoV-2 replication while uncovering the nuanced regulatory roles of NSP7 and NSP8. The researchers demonstrated that NSP12 can independently carry out RNA polymerase activity but requires both cofactors to function optimally. The inhibitory effect of individual cofactors highlights a potential vulnerability in the viral replication machinery, which could be exploited for therapeutic purposes.
Targeted antiviral strategies disrupting NSP12’s interactions with NSP7 and NSP8 could pave the way for novel treatments. By preventing the assembly of the RdRp holoenzyme, such therapies could effectively halt viral replication, providing a new line of defense against coronaviruses.
The study findings were published in the peer-reviewed journal: Molecular Therapy - Nucleic Acids.
https://www.sciencedirect.com/science/article/pii/S216225312500006X
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/mutations-of-sars-cov-2-nsp12-nsp8-interface-affect-viral-replication
https://www.thailandmedical.news/news/murine-study-shows-that-sars-cov-2-nsp12-suppresses-mitochondrial-function-in-heart-tissues
https://www.thailandmedical.news/news/south-korean-ferret-study-finds-sars-cov-2-nsp12-p323l-and-g671s-mutations-causes-enhanced-transmissibility-and-virus-replication-in-upper-airway
https://www.thailandmedical.news/news/covid-19-news-uk-study-finds-that-sars-cov-2-nsp12-associates-with-tric-and-the-p323l-substitution-acts-as-a-host-adaption
https://www.thailandmedical.news/news/breaking-news-u-s-researchers-discover-1-4-kb-long-sequence-in-sars-cov-2-nsp12-and-nsp13-regions-that-facilitates-viral-rna-packaging
https://www.thailandmedical.news/news/study-uncovers-that-sars-cov-2-nsp12-proteins-play-a-key-role-in-virus-replication-and-splicing-regulation
https://www.thailandmedical.news/articles/coronavirus
