Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 21, 2024 4 months, 3 weeks, 1 day, 56 minutes ago
Medical News: Researchers from the CNRS/Université de Lyon, France, and ETH Zurich, Switzerland, have uncovered vital insights into the SARS-CoV-2 ORF7b protein. This enigmatic component of the virus plays a significant role in host-virus interactions but has largely escaped detailed analysis due to its unique structural properties. The team applied advanced biochemical and solid-state NMR techniques to decode its structure and interactions, revealing its potential as a target for further studies.
 New Insights into the Role of SARS-CoV-2 ORF7b
New Insights into the Role of SARS-CoV-2 ORF7b
This
Medical News report dives into the structural characteristics and biological relevance of ORF7b, shedding light on how it interacts with human proteins and potentially influences COVID-19 pathology.
ORF7b: The Lesser-Known Player in SARS-CoV-2
The genome of SARS-CoV-2 is a masterpiece of viral evolution, encoding 27 proteins. Among these are nine accessory proteins, including ORF7b, a small membrane protein with a structure that has long puzzled scientists. While ORF7b isn’t essential for viral replication, it may affect how the virus evades the immune system and interacts with human cellular machinery.
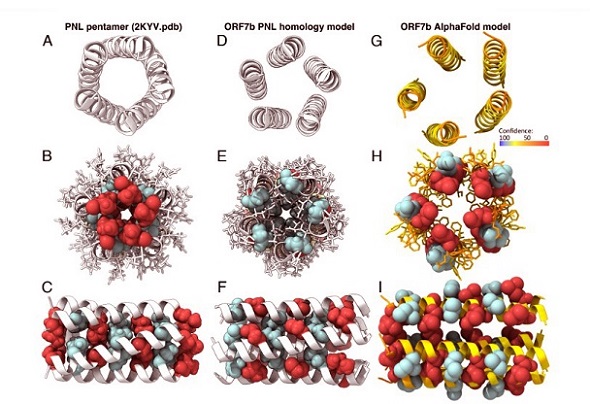
ORF7b is notably small, consisting of only 43 amino acids, yet it is highly conserved between SARS-CoV and SARS-CoV-2. This protein also shares significant similarity with bat coronaviruses, hinting at its evolutionary importance. Its key feature is a transmembrane (TM) domain, which appears to mediate both its structural stability and interactions with other proteins.
Structural Insights: What Makes ORF7b Unique?
Using advanced modeling techniques, researchers identified a leucine zipper motif in the transmembrane region of ORF7b. This motif, commonly found in soluble proteins, enables interaction and multimerization. However, its presence in a membrane-bound protein like ORF7b is rare, making it a subject of interest.
Experiments confirmed that ORF7b primarily adopts an α-helical structure, forming heterogeneous multimers. These multimers are crucial for the protein’s function, as they facilitate interactions with specific human proteins like E-cadherin and phospholamban (PLN).
ORF7b and Human Proteins: Potential Disruptors of Cellular Functions
The team found compelling evidence that ORF7b interacts with human proteins containing transmembrane leucine zipper motifs. Two standout interactions were observed:
-E-Cadherin: This protein is vital for cell adhesion. ORF7b's interaction with E-cadherin may disrupt cellular connections, which could contribute to the severe tissue damage seen in COVID-19 patients.
-Phospholamban (PLN): Critical for regulating heart contractions, PLN's interaction with ORF7b raises concerns about potential cardiovascular complications linked to COVID-19.
These findings place ORF7b as a hypothetical interferer in
cellular processes that rely on transmembrane multimerization domains.
Challenges in Analyzing ORF7b
Despite its significance, ORF7b is notoriously difficult to study. Its small size, hydrophobicity, and membrane-bound nature have hindered traditional structural biology approaches. Researchers overcame these challenges using solid-state NMR, which allowed for detailed structural analysis even in lipid environments.
Interestingly, ORF7b forms higher-order multimers that do not exhibit a fixed structure, suggesting functional flexibility. The protein’s interactions seem to depend on its ability to adapt to different molecular environments, further complicating its study.
Broader Implications of the Findings
The discovery of ORF7b’s interactions with E-cadherin and PLN adds another layer to our understanding of how SARS-CoV-2 affects the human body. By targeting critical cellular functions, ORF7b may contribute to the virus's ability to cause severe disease. This highlights the importance of accessory proteins in the broader context of viral pathogenesis and immune evasion.
These findings could pave the way for new therapeutic strategies. For instance, drugs designed to block ORF7b's interactions with human proteins could potentially mitigate some of the more severe symptoms of COVID-19.
Conclusion: A Step Forward in SARS-CoV-2 Research
The study offers a detailed glimpse into the structure and function of ORF7b, underscoring its role as more than a simple accessory protein. By interacting with key human proteins, ORF7b could influence a range of cellular processes, from adhesion to cardiac function. These findings provide a foundation for future research aimed at exploiting this protein's vulnerabilities.
As scientists continue to decode the mysteries of SARS-CoV-2, ORF7b emerges as a promising target for interventions that could reduce the severity of COVID-19. Understanding its structure and interactions is not just an academic exercise but a potential pathway to new treatments.
The study findings were published in the peer-reviewed journal: Proceedings of the National Academy of Sciences (PNAS).
https://www.pnas.org/doi/epub/10.1073/pnas.2407731121
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-news-pittsburg-study-shows-that-sars-cov-2-orf7b-protein-induces-lung-injury-via-c-myc-mediated-apoptosis-and-ferroptosis
https://www.thailandmedical.news/news/covid-19-news-sars-cov-2-orf7a-blocked-autophagy-flux-by-intervening-in-the-fusion-between-autophagosome-and-lysosome-to-promote-viral-infection
