New Insights on COVID-19 Fusion Peptides Could Lead to Better Antiviral Treatments
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 22, 2024 1 year, 2 months, 2 days, 6 hours, 23 minutes ago
Medical News: Researchers from Monash University-Australia, the University of Alabama at Birmingham-USA, and the Australian Nuclear Science and Technology Organisation (ANSTO) have discovered new details about how certain parts of the COVID-19 virus interact with the body. The study sheds light on how specific parts of the virus, called fusion peptides, behave in different environments. This could lead to more effective antiviral therapies targeting viral entry into human cells.
 Graphical Abstract: New Insights on COVID-19 Fusion Peptides Could Lead to Better Antiviral Treatments
What Are Fusion Peptides?
Graphical Abstract: New Insights on COVID-19 Fusion Peptides Could Lead to Better Antiviral Treatments
What Are Fusion Peptides?
Fusion peptides are small sections of a virus that help it fuse with the membrane of a human cell. This is a critical step in the infection process because, without this fusion, the virus can't inject its genetic material into the cell and begin to replicate. Understanding how these fusion peptides work can provide valuable clues on how to stop the virus from infecting human cells.
In this
Medical News report, we focus on the fusion peptides of the COVID-19 virus, specifically a section called the N-terminal fusion peptide. The study looked at how acetylation, a chemical modification, affects these peptides’ ability to interact with lipid bilayers - structures similar to human cell membranes.
The Study Approach
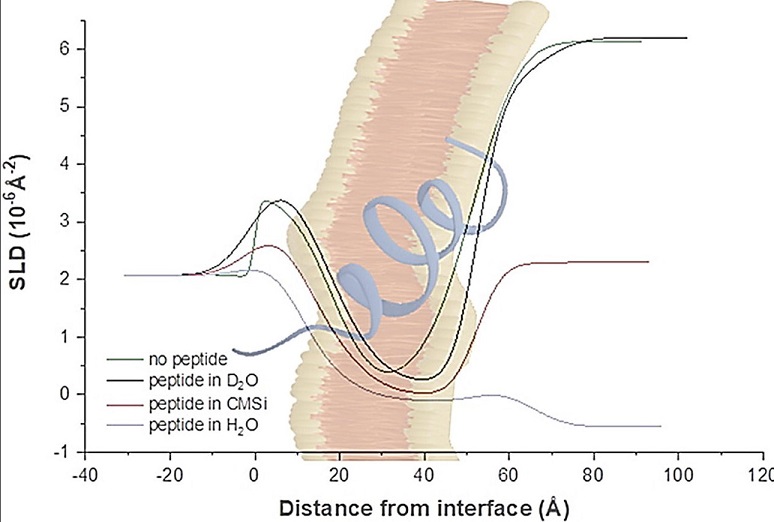
The researchers used advanced techniques like Neutron Reflectometry (NR) and Quartz Crystal Microbalance with Dissipation (QCM-D) to closely observe how the viral fusion peptides behave when they come into contact with lipid bilayers. These bilayers mimic the environment of human cell membranes. The research team studied two different types of bilayers:
-Liquid-Ordered Domains: More structured, cholesterol-rich areas of the membrane, which tend to be more stable.
-Liquid-Disordered Domains: Less structured, more fluid areas of the membrane, which are easier to penetrate.
Interestingly, the unmodified fusion peptides (those without acetylation) did not show significant interaction with either type of membrane. However, when these peptides were acetylated, their behavior changed dramatically.
Key Findings
The study’s key discovery was that the acetylated COVID-19 fusion peptides showed a much stronger interaction with the cholesterol-rich, liquid-ordered domains of the lipid bilayers. This suggests that acetylation is an important factor in helping the virus bind to the cell membrane. The acetylated peptides were able to change the structure of the bilayer, making it thicker and more rigid.
In contrast, the unacetylated fusion peptides had very little effect on the membranes, indicating that they may not be as effective in promoting the fusion necessary for viral entry.
This acetylation process appears to influence how the peptides interact with tightly packed lipids such as
sphingomyelin and cholesterol, which are abundant in certain parts of human cell membranes known as "lipid rafts." These lipid rafts are thought to be hotspots for viral entry because they cluster important proteins that viruses need to hijack for infection.
Why Is This Important?
The results of this study provide valuable insights into how the coronavirus enters human cells. By showing that acetylation plays a key role in enabling the virus to fuse with the cell membrane, this research opens the door for the development of new therapies.
Dr. Izabela Miłogrodzka from Monash University and her colleagues suggest that targeting the acetylation process or the lipid rafts could lead to new ways to prevent the virus from infecting cells. This could be particularly useful in developing antiviral drugs that could block the initial stage of infection, making it harder for the virus to spread within the body.
Potential Applications
The findings from this study could have broader implications beyond just COVID-19. Other viruses, including influenza and HIV, also rely on fusion peptides to enter human cells. By applying what we’ve learned about COVID-19 fusion peptides, researchers might be able to develop new antiviral strategies for these other viruses as well.
For example, similar research has shown that modifying fusion peptides can either increase or decrease their ability to fuse with cell membranes. This suggests that chemical modifications like acetylation could be a general approach to controlling viral infection.
Conclusion
This study represents a significant step forward in understanding the mechanisms of viral infection. By focusing on the role of acetylation in COVID-19 fusion peptides, the researchers have uncovered new details about how the virus interacts with the cell membrane. These findings could help in developing new antiviral therapies, not only for COVID-19 but also for other viruses that use similar mechanisms to infect human cells.
The study findings were published in the peer-reviewed Journal of Colloid and Interface Science.
https://www.sciencedirect.com/science/article/pii/S0021979724024664
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/cd81-fusion-protein-enhances-sars-cov-2-spike-trafficking-and-immune-response
https://www.thailandmedical.news/news/key-positive-residues-in-sars-cov-2-fusion-domain-are-crucial-for-membrane-fusion
