New Treatment Protocol For Previously Untreatable Multiple Myeloma Using Selinexor
Source: Thailand Medical News Aug 22, 2019 5 years, 8 months, 4 days, 21 hours, 46 minutes ago
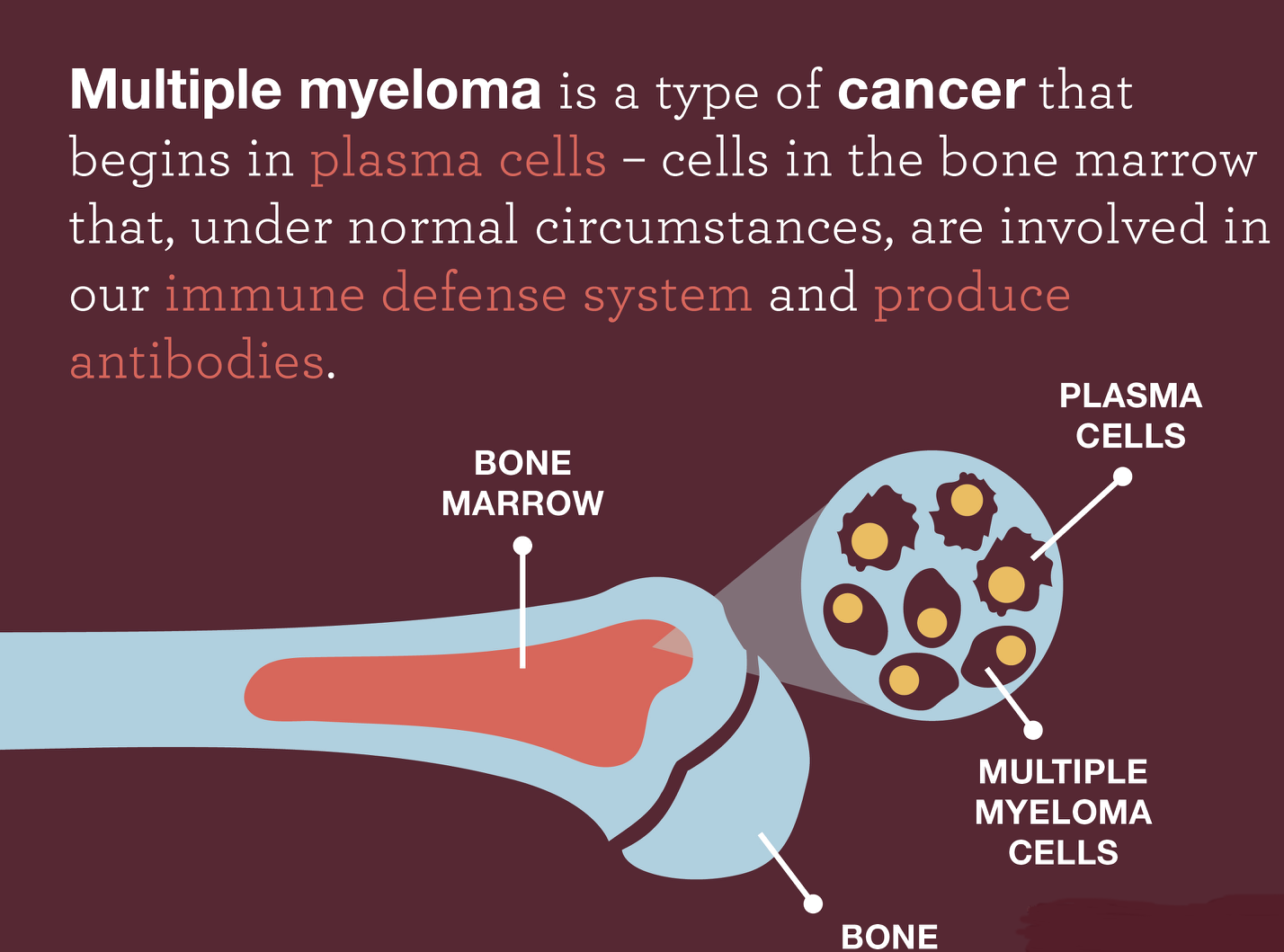
Multiple myeloma is a cancer that forms in a type of white blood cell called a plasma cell. It is the second form of mostly diagnosed type of blood cancer and very often becomes resistant to various existing treatments and become untreatable.
Fortunately, Mount Sinai researchers have found a new type of therapy to be effective for patients with this particular type of blood bone marrow cancer that is resistant to several standard therapies.
This team conducted a trial to test selinexor with dexamethasone, a combination that significantly put down the cancer in more than 40 per cent of patients, including two patients who went into complete remission. Proteins and messenger RNAs play an important part of cancer cell growth, and selinexor has an remarkable mechanism that blocks the export of protein and messenger RNAs from the nucleus of the cancer cell to the cytoplasm, causing the cancer cell to die. This therapy caused at least a minimal response in almost 45 percent of patients who had multiple myeloma, a cancer of a type of white blood cell called a plasma cell.
Senior Author, Professor Dr Sundar Jagannath, commented in an exclusive interview with Thailand Medical News "This trial proved that a new, first-in-class drug with a new mechanism of action can kill a patient's cancer cells. This proved that the drug worked in patients who had exhausted every other treatment and who would have been placed on palliative care otherwise."
The STORM Part 2 clinical trial studied the response of 122 patients taking selinexor and dexamethasone, both oral drugs, in trials across the America and Europe.
Participants with multiple myeloma generally saw a response to the drugs within one or two months. While there was no organ toxicity, side effects included low blood count without bleeding, nausea, vomiting, lack of appetite or fatigue.
Typically, a huge proportion of patients have resistance to the standard drugs used in the treatment of multiple myeloma, and the overall survival in these patients is short, sometimes less than three months.
Selinexor was approved by the FDA for patients resistant to multiple therapies in early July 2019.
Selinexor is also being investigated and researched in trials patients with multiple myeloma but combination with other approved multiple myeloma drugs, and also being researched in usage as a possible treatment drug for other malignancies such lymphoma and ovarian cancer.
