Ohio Study Finds Distinct SARS-CoV-2 Specific NLRP3 And IL-1β Responses In T Cells Of Aging COVID-19 Patients, Reflecting Dysregulated Immune Response!
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 22, 2023 1 year, 6 months, 3 weeks, 6 days, 17 hours, 11 minutes ago
COVID-19 News: Since its emergence in late 2019, the COVID-19 pandemic has continued to impact populations worldwide. COVID-19, caused by the SARS-CoV-2 virus, presents with a wide spectrum of clinical manifestations, ranging from asymptomatic or mild infections to severe cases characterized by cytokine storms, acute respiratory distress syndrome (ARDS), and, in some instances, fatal outcomes. While it is well-established that advanced age is a significant risk factor for severe disease, the precise immunological factors driving these outcomes in elderly individuals remain poorly understood.
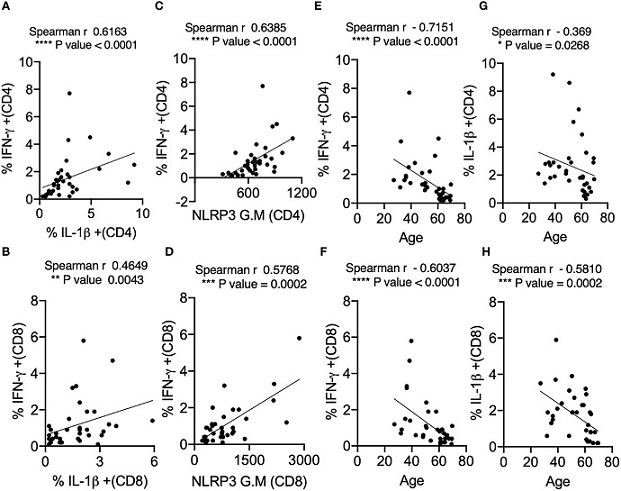 IL-1β expression positively correlates with IFN-γ and NLRP3 expression in SPIKE-activated T cells in COVID+ patients. Spearman Correlation (r) analysis showing positive correlation between IL-1β and IFN-γ in CD4+ T cells (A) and CD8+ T cells (B), and NLRP3 and IFN-γ in CD4+ T cells (C), and CD8+ T cells (D). Negative correlation between cytokines and age in CD4+ T cells (E, G) and CD8+ T cells (F, H). *P<0.05, **<0.005, ***<0.0005, ****<0.00005.
IL-1β expression positively correlates with IFN-γ and NLRP3 expression in SPIKE-activated T cells in COVID+ patients. Spearman Correlation (r) analysis showing positive correlation between IL-1β and IFN-γ in CD4+ T cells (A) and CD8+ T cells (B), and NLRP3 and IFN-γ in CD4+ T cells (C), and CD8+ T cells (D). Negative correlation between cytokines and age in CD4+ T cells (E, G) and CD8+ T cells (F, H). *P<0.05, **<0.005, ***<0.0005, ****<0.00005.
In a new study conducted by researchers at Case Western Reserve University in Cleveland, Ohio, and the Louis Stokes Cleveland Veterans Affairs Medical Center, a unique insight into the immune responses of aging COVID-19 patients has emerged. The study reveals distinct alterations in T cell responses among individuals aged 61 and older, even in cases of mild COVID-19. Specifically, when exposed to SARS-CoV-2 peptides in vitro, CD4+ and CD8+ T cells in individuals aged 61 and above displayed reduced production of critical immune molecules such as interferon-gamma (IFN-γ) and interleukin-1 beta (IL-1β). These findings are indicative of an intrinsic dysregulation in the immune response of older individuals to SARS-CoV-2, even in the absence of severe disease.
Understanding the immune mechanisms underlying these age-related differences is crucial for developing targeted therapeutic interventions and optimizing vaccination strategies for the elderly population.
The Role of T Cells in COVID-19
To comprehend the significance of the study's findings, it is essential to first acknowledge the role of T cells in the context of COVID-19. The SARS-CoV-2 virus primarily infects the epithelial cells of the respiratory tract and lungs. The severity of infection is often associated with viral presence in the lungs, which can lead to severe respiratory complications as shown in various studies and past
COVID-19 News reports.
T cells, a vital component of the immune system, play a pivotal role in the body's defense against viral infections. Specifically, T helper 1 (Th1) and T follicular helper (TFH) CD4+ T cells, along with CD8+ T cells, are responsible for orchestrating immune responses against SARS-CoV-2. In mild cases of COVID-19, patients often exhibit an early induction of IFN-γ-secreting T cells, indicating a robust antiviral response.
Studies in animal models have underscored the importance of CD8+ and CD4+ T cells in clearing the virus, limiting disease severity, and facilitating recovery post-infec
tion. Furthermore, the suppression of IFN-γ has been linked to reduced survival rates following viral challenge.
In severe COVID-19 cases, a distinct pattern emerges, characterized by delayed viral clearance, hyperactivation of the immune system (referred to as a "cytokine storm"), and elevated levels of pro-inflammatory cytokines like IFN-α, IFN-γ, and tumor necrosis factor-alpha (TNF-α). This cascade of events contributes to the severe clinical outcomes observed in these patients.
Mapping T Cell Responses to SARS-CoV-2
To better understand the immune response to SARS-CoV-2, researchers have mapped the T cell response to various viral proteins, including the spike (S), nucleocapsid (N), and envelope (E) proteins. These studies have identified dominant epitopes recognized by CD4+ and CD8+ T cells, primarily originating from S, N, and E proteins.
Remarkably, even individuals with no prior exposure to SARS-CoV-2 may possess pre-existing memory T cells that cross-react with the virus. This cross-reactivity could partially explain why some individuals experience milder COVID-19 symptoms.
Aging and the Immune System
Human aging is associated with systemic inflammation and a decline in immune function, a phenomenon often referred to as "inflammaging" and "immunosenescence." Several factors contribute to these age-related changes, including a reduction in the number of naïve CD4+ and CD8+ T cells and alterations in regulatory T cells (Tregs), which play a role in limiting immune responses.
Additionally, aging is marked by an increase in terminally differentiated CD8+ T cells and proliferative senescent T cells, both of which exhibit pro-inflammatory behavior. Co-stimulatory molecules like CD28 decline with age, while CD57 expression increases, resulting in non-proliferative T cells. This shift towards a pro-inflammatory state can lead to an impaired anti-inflammatory response in older individuals.
In the context of SARS-CoV-2 infection, these age-related immune system changes can diminish the ability of T cells to mount a robust immune response to the virus. The study conducted at Case Western Reserve University aimed to uncover the specific alterations in T cell responses among older COVID-19 patients, shedding light on the unique challenges faced by this vulnerable population.
Key Findings
The study yielded several critical findings:
T Cell Alterations in Aging Patients: Even among COVID-19 patients with mild symptoms, individuals aged 61 and older displayed intrinsic changes in T cell responses. When exposed to SARS-CoV-2 peptides in vitro, CD4+ and CD8+ T cells from this age group exhibited reduced capacity to produce IFN-γ and IL-1β, two crucial immune molecules.
Increased PD-1 Expression: A higher frequency of PD-1+ cells was observed in aged individuals upon SARS-CoV-2 peptide stimulation. This suggests potential T cell exhaustion, which could contribute to the impaired immune responses seen in older COVID-19 patients.
Diminished IFN-γ/PD-1 Ratios: Older individuals also exhibited significantly reduced IFN-γ/PD-1 ratios among T lymphocytes upon SARS-CoV-2 peptide stimulation. This further supports the notion of T cell exhaustion in this age group.
Reduced IL-1β Expression: Impaired T cell IL-1β expression was noted in aged individuals, coinciding with reduced levels of the NLRP3 protein in T lymphocytes. Interestingly, these alterations were not observed in monocytes, suggesting a T cell-specific phenomenon.
Further Insights And Implications
The study's findings provide valuable insights into the immune response of older individuals to SARS-CoV-2 infection. Notably, these insights are not limited to severe cases but extend to mild COVID-19 cases in the elderly population.
Several key points emerge from the study's discussion:
Unique T Cell Alterations: The reduced production of IFN-γ and IL-1β in T cells from older COVID-19 patients is a novel discovery. These alterations appear to be distinct from T cell exhaustion, as intrinsic differences were observed even without stimulation with SARS-CoV-2 peptides.
Potential Impairment in Antigen Presentation: While the study focused on T cell responses, the role of antigen presentation, co-stimulation, and co-inhibition effects on T cell NLRP3 and cytokine expression warrant further investigation. Impaired antigen presentation by aging T cells could be one mechanism contributing to the observed effects.
Differences Between Innate and Adaptive Immune Responses: The study highlights the distinction between innate hyperinflammatory responses and antigen-specific IL-1β responses in blood T cells. Excessive NLRP3/IL-1β levels may be pathogenic, but suboptimal levels could pose a risk for secondary infections in the elderly.
Clonal Expansion Hypothesis: The reduced cytokine-expressing T cells in response to SARS-CoV-2 peptides in aged COVID-19 patients could be attributed to reduced clonal expansion or a lower frequency of clonally expanded effector or memory cells. Further research is needed to explore this possibility.
Role of IL-1β in Memory T Cell Responses: The study suggests that IL-1β plays a vital role in antigen-specific IFN-γ responses and T cell-intrinsic signaling, particularly in memory CD4+ T cells. Understanding the implications of impaired IL-1β responses in aging during SARS-CoV-2 infection is essential.
Conclusion
The study conducted at Case Western Reserve University and the Louis Stokes Cleveland Veterans Affairs Medical Center has provided valuable insights into the immune responses of aging COVID-19 patients. By uncovering distinct alterations in T cell cytokine production and NLRP3 expression among individuals aged 61 and older, even in cases of mild COVID-19, this research has highlighted the complexity of immune dysregulation in the elderly population.
These study findings hold significant implications for the development of therapeutic interventions and vaccination strategies tailored to older individuals. A deeper understanding of the age-related immune changes observed in this study will contribute to more effective approaches to combat COVID-19 in the vulnerable elderly population, ultimately reducing the burden of the disease on healthcare systems and improving patient outcomes. Further research is warranted to validate and expand upon these findings, potentially unlocking new avenues for targeted treatments and preventive measures against COVID-19.
The study findings were published in the peer reviewed journal: Frontiers In Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1231087/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
