Oklahoma Study Involving Feline Model Shows That SARS-CoV-2 Causes Alterations Of Whole-Body Glucose Metabolism
Nikhil Prasad Fact checked by:Thailand Medical News Apr 20, 2024 1 year, 5 days, 22 hours, 54 minutes ago
COVID-19 News: SARS-CoV-2, the virus responsible for COVID-19, has proven particularly lethal for individuals with pre-existing metabolic and cardiovascular conditions. Elevated blood glucose levels have been associated with increased mortality in COVID-19 patients. However, the precise mechanisms linking SARS-CoV-2 infection and alterations in glucose metabolism remain poorly understood. A recent study conducted at Oklahoma State University-USA that is covered in this
COVID-19 News report, aimed to investigate these mechanisms using a feline model of SARS-CoV-2 infection.
 Feline Model Shows That SARS-CoV-2 Causes Alterations Of Whole-Body Glucose Metabolism
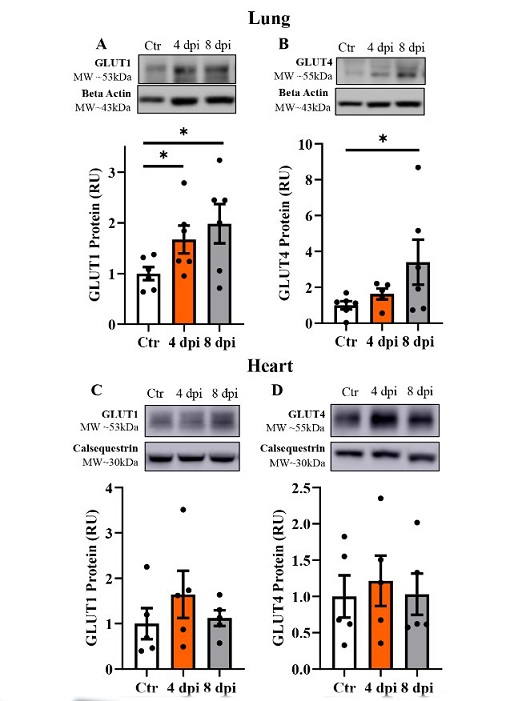
SARS‐CoV‐2 infection induced increased protein levels of glucose transporter (GLUT) in 502 the lung and skeletal muscle, but not in the heart. Top panels: representative Western blot 503 from total lysate; beta actin or calsequestrin were used as a loading control; representative 504 bands were obtained from the same membrane. Bottom panels: Mean ± SE of relative total 505 protein content of basal GLUT1 and insulin‐sensitive GLUT4 in the lung (A‐B), heart (C‐D) of control (Ctr) and infected cats, respectively; n=6/group; * p<0.05.
Background and Rationale
Feline Model Shows That SARS-CoV-2 Causes Alterations Of Whole-Body Glucose Metabolism
SARS‐CoV‐2 infection induced increased protein levels of glucose transporter (GLUT) in 502 the lung and skeletal muscle, but not in the heart. Top panels: representative Western blot 503 from total lysate; beta actin or calsequestrin were used as a loading control; representative 504 bands were obtained from the same membrane. Bottom panels: Mean ± SE of relative total 505 protein content of basal GLUT1 and insulin‐sensitive GLUT4 in the lung (A‐B), heart (C‐D) of control (Ctr) and infected cats, respectively; n=6/group; * p<0.05.
Background and Rationale
The study of glucose metabolism during SARS-CoV-2 infection is crucial due to its potential impact on disease severity and patient outcomes. Previous research has shown that glucose transport pathways are essential for the replication of other RNA viruses. Therefore, it was hypothesized that SARS-CoV-2 infection could lead to changes in cellular and whole-body glucose metabolism.
Experimental Design
Specific pathogen-free domestic cats were intratracheally inoculated with Wild-type SARS-CoV-2 or a control vehicle and then assessed at 4- and 8-days post-inoculation (dpi). Various parameters including blood glucose, cortisol, ketones, insulin, angiotensin 2 concentrations, and RNA expression levels were measured. Protein levels of glucose transporters (GLUTs) and AMP-activated protein kinase (AMPK) were also analyzed in different tissues.
Results and Findings
The study yielded several noteworthy results regarding whole-body glucose metabolism in the context of SARS-CoV-2 infection:
-Blood Glucose and Cortisol Levels
At both 4 and 8 dpi, infected cats exhibited elevated levels of blood glucose, indicating a state of hyperglycemia. This finding is consistent with clinical observations in COVID-19 patients and suggests a potential link between viral infection and dysregulation of glucose homeostasis. Concurrently, cortisol concentrations were also elevated in infected cats. Cortisol, a stress hormone, plays a role in glucose metabolism by promoting gluconeogenesis in the liver and inhibiting insulin secretion. The observed increase in cortisol levels may indicate a stre
ss response triggered by SARS-CoV-2 infection, contributing to hyperglycemia.
-Ketones, Insulin, and Angiotensin 2 Concentrations
Contrary to the changes in blood glucose and cortisol, levels of ketones, insulin, and angiotensin 2 remained unchanged throughout the experimental timeline. Ketones are typically elevated in severe cases of diabetes but were not significantly altered in this pre-diabetic state observed in the infected cats.
Similarly, insulin levels did not decrease following infection, suggesting alternative mechanisms driving hyperglycemia beyond insulin deficiency. Additionally, angiotensin 2 concentrations, implicated in COVID-19 pathogenesis, did not show significant changes, indicating a more nuanced relationship between the virus and the renin-angiotensin system.
-Tissue-Specific Protein Expression
In the lung, protein levels of glucose transporter 1 (GLUT1) were significantly increased at both 4 and 8 dpi, indicating enhanced glucose uptake and utilization in response to viral infection. This upregulation of GLUT1 may facilitate the metabolic demands associated with viral replication in lung tissue. Interestingly, GLUT4 levels were only upregulated at 8 dpi, suggesting a delayed but specific response in insulin-sensitive glucose transport.
Contrastingly, in the heart, there were no significant changes observed in GLUT1 and GLUT4 protein levels following infection. This differential response between lung and heart tissues highlights the tissue-specific nature of metabolic adaptations during SARS-CoV-2 infection.
-AMPK Activation
AMP-activated protein kinase (AMPK), a key regulator of cellular energy homeostasis, showed increased activation in the heart tissue of infected cats. This activation of AMPK indicates a metabolic shift aimed at meeting the heightened energy demands associated with viral infection. The activation of AMPK may play a role in modulating glucose transport and utilization to support the metabolic requirements of the infected tissues.
Implications of Findings
The results suggest a complex interplay between SARS-CoV-2 infection and glucose metabolism. Elevated blood glucose and cortisol levels indicate a stress-induced hyperglycemic state, potentially driven by the immune response to viral infection. Tissue-specific alterations in GLUT protein levels and AMPK activation highlight the dynamic metabolic adaptations occurring in response to SARS-CoV-2, particularly in tissues directly affected by the virus.
Significance of the Findings
These findings have significant implications for understanding the metabolic consequences of SARS-CoV-2 infection. The observed alterations in glucose metabolism shed light on potential therapeutic targets for managing hyperglycemia and metabolic complications in COVID-19 patients. Targeting key regulators such as GLUTs and AMPK could offer novel strategies to mitigate the metabolic impact of viral infection, potentially improving patient outcomes and prognosis.
The findings suggest that SARS-CoV-2 infection induces metabolic changes primarily in the lung and heart tissues to support viral replication. The activation of AMPK and alterations in GLUT protein levels indicate a shift in cellular metabolism to meet the increased energy demands during infection. These insights contribute to a better understanding of COVID-19 pathophysiology and may lead to the development of targeted therapeutic strategies.
This study highlights the importance of investigating metabolic pathways in viral infections such as COVID-19. The use of a feline model provides valuable insights into whole-body glucose metabolism alterations during SARS-CoV-2 infection. Understanding these mechanisms could pave the way for novel therapeutic interventions targeting metabolic pathways to improve patient outcomes.
Future Directions and Implications
Further research is needed to elucidate the precise molecular mechanisms linking SARS-CoV-2 infection and alterations in glucose metabolism. Targeting specific components of these pathways could offer new avenues for therapeutic development. The translational relevance of the feline model underscores its potential for future studies exploring metabolic therapeutics for COVID-19 and related conditions.
Conclusion
In summary, the results of the study provide valuable insights into the alterations in whole-body glucose metabolism induced by SARS-CoV-2 infection in a feline model. The findings underscore the complex interactions between viral infection, stress response, and metabolic pathways, highlighting the need for further research to elucidate the underlying mechanisms and explore targeted interventions for COVID-19-related metabolic disturbances.
The study findings were published in the peer reviewed journal: American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.
https://journals.physiology.org/doi/abs/10.1152/ajpregu.00228.2023
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
