Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 09, 2024 9 months, 5 hours, 45 minutes ago
COVID-19 News: Researchers from Hokkaido University-Japan and the Japan Agency for Medical Research and Development have made a significant discovery about the Omicron variant of SARS-CoV-2. Identified first in South Africa in 2021, this variant of the virus has shown an increased ability to bind to human cells, specifically through its interaction with a receptor called hACE2. This
COVID-19 News report explores the findings and implications of this enhanced binding, particularly its interaction with actin, a crucial protein in human cells.
 Omicron variant shows enhanced binding to human cells and actin
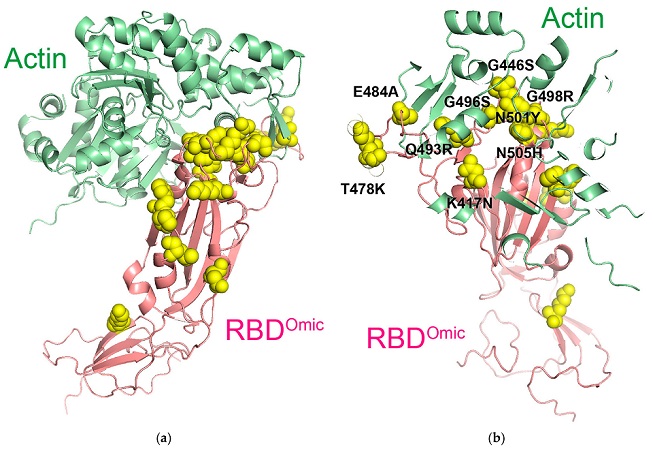
Key amino acids in the predicted complex of omicron RBD and β-actin. (a) Predicted protein complex of the omicron RBD (RBDOmic; light magenta) and β-actin (light green). Yellow spheres indicate the missense mutations in the omicron RBD. (b) Enlarged view of the complex of omicron RBD and actin with higher transparency. Black letters indicate the clustered missense amino acids in the RBD within 6 Å from the actin surface
Key Findings of the Study
Omicron variant shows enhanced binding to human cells and actin
Key amino acids in the predicted complex of omicron RBD and β-actin. (a) Predicted protein complex of the omicron RBD (RBDOmic; light magenta) and β-actin (light green). Yellow spheres indicate the missense mutations in the omicron RBD. (b) Enlarged view of the complex of omicron RBD and actin with higher transparency. Black letters indicate the clustered missense amino acids in the RBD within 6 Å from the actin surface
Key Findings of the Study
The study, led by Ai Fujimoto, Haruki Kawai, Rintaro Kawamura, and Akira Kitamura, revealed that the Omicron variant’s spike protein has undergone multiple amino acid mutations. These mutations significantly enhance its binding affinity to hACE2, a receptor on the surface of human cells. The spike protein's receptor-binding domain (RBD) is the key part that interacts with hACE2, facilitating the virus’s entry into the cells and initiating infection. This article aims to provide an easy-to-understand summary of these findings.
Increased Affinity of Omicron for hACE2
The team used a technique called fluorescence cross-correlation spectroscopy (FCCS) to compare the binding strength of the Omicron RBD with that of the original SARS-CoV-2 strain, known as the Wuhan-Hu-1 strain. Their experiments showed that the Omicron RBD binds more strongly to hACE2, suggesting why this variant might spread more easily between people.
The researchers discovered that specific mutations, such as T478K, Q493K, and Q498R, contribute to this increased binding affinity. These mutations alter the structure of the RBD in a way that makes it easier for the virus to latch onto hACE2.
Interaction with Actin
Beyond hACE2, the study found that the Omicron RBD also binds to actin, a protein that plays a vital role in maintaining cell structure and function. Actin is a part of the cytoskeleton, which helps cells maintain their shape, move, and divide. The researchers identified two forms of actin, β-actin and γ-actin, as specific binding partners of the Omicron RBD.
Using mass spectrometry and western blotting techniques, they confirmed that these forms of actin bind specifically to the Omicron RBD but not to the RBD of the original strain. This sugg
ests that the Omicron variant may influence the behavior of human cells differently from earlier variants.
Visualizing the Interaction
To understand how the Omicron RBD interacts with actin, the researchers used confocal fluorescence microscopy. They tagged the RBD with a fluorescent marker and observed its behavior in human cells. The images showed that while the Omicron RBD does not bind to structured actin filaments, it does interact with diffuse or monomeric actin, which is not part of the cell's structural framework but still crucial for various cellular functions.
Implications for Virus Spread and Immune Response
The enhanced binding of the Omicron variant to hACE2 and its interaction with actin could explain its higher transmissibility. By binding more effectively to hACE2, the virus can enter cells more efficiently, potentially leading to faster spread. Additionally, the interaction with actin might help the virus manipulate the host cell's environment to its advantage, aiding in replication and evasion of the immune system.
These findings have important implications for public health, as they highlight the need for continued monitoring of SARS-CoV-2 mutations and their effects on virus behavior and vaccine effectiveness.
Conclusion
In conclusion, the study by researchers from Hokkaido University and the Japan Agency for Medical Research and Development provides crucial insights into the Omicron variant's enhanced ability to bind to human cells. This increased affinity for hACE2, coupled with its interaction with actin, suggests that Omicron can spread more easily and potentially evade immune responses more effectively than previous variants. Understanding these mechanisms is vital for developing effective treatments and vaccines to combat COVID-19.
The study findings were published in the peer-reviewed journal: Cells.
https://www.mdpi.com/2073-4409/13/16/1318
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-news-scientists-discover-that-spike-protein-of-omicron-and-its-sub-lineages-binds-with-actin
https://www.thailandmedical.news/news/interleukin-1-beta-inhibits-sars-cov-2-spread-by-preventing-cell-fusion-through-actin-bundle
