Oxford Study Shows That SARS-CoV-2 Infection Induces a Prolonged Pro-Inflammatory Transcriptional Profile With Far-Reaching Health Implications
Nikhil Prasad Fact checked by:Thailand Medical News Team Sep 15, 2023 2 years, 3 months, 1 week, 5 days, 5 hours, 39 minutes ago
COVID-19 News: The COVID-19 pandemic has been a global healthcare crisis of unprecedented proportions, leading to extensive research efforts to understand the immune response to the SARS-CoV-2 virus and its impact on individuals. While significant progress has been made in understanding the acute phase of the disease, there remains a dearth of knowledge concerning the long-term effects of SARS-CoV-2 infection on patients.
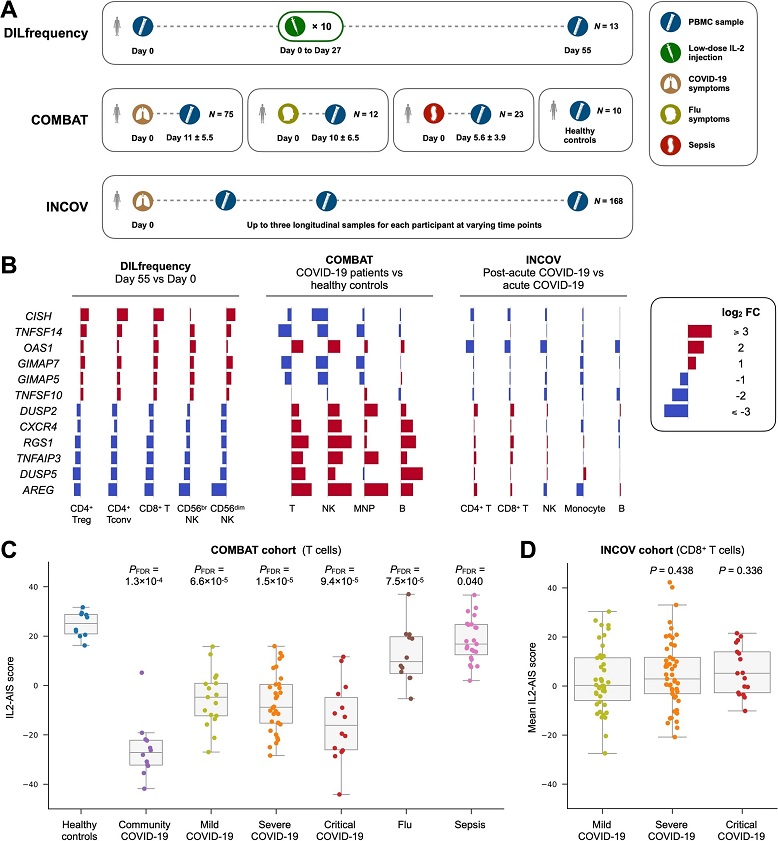 Low-dose IL-2 immunotherapy and SARS-CoV-2 infection induce opposite transcriptional changes in immune cells. A Overview of the DILfrequency, COMBAT and INCOV cohorts. B Differential expression induced by low-dose IL-2 immunotherapy (DILfrequency) and SARS-CoV-2 infection (COMBAT and INCOV) on the top six up and downregulated genes from the anti-inflammatory gene expression signature induced by low-dose IL-2 treatment (IL2-AIS), previously identified in the DILfrequency cohort. Data shown depicts the log2 fold change (FC) values in each cohort and were calculated in the different immune subsets identified in the respective study. P values and additional IL2-AIS constituent genes are shown in Additional file 3: Fig. S1. C Distribution of the IL2-AIS scores in T cells from each participant group in the COMBAT cohort. Data was stratified by disease group and COVID-19 disease severity. P values were calculated by comparing each patient group with healthy controls using a two-sided Mann–Whitney U test followed by FDR adjustment. D Mean IL2-AIS scores in CD8+ T cells from each participant group in the INCOV cohort from samples collected during the first 14 days after symptoms onset. Data was stratified by COVID-19 disease severity. P values are calculated by comparing the severe or critical COVID-19 group with the mild COVID-19 group using a two-sided Mann–Whitney U test. In C and D, each box ranges from the first quartile (Q1) to the third quartile (Q3), with a central line indicating the median. Treg, regulatory T cells. Tconv, conventional T cells. MNP, mononuclear phagocytes. The IL2-AIS scores were derived from pseudo-bulk samples aggregated from single-cell RNAseq data of PBMCs
Low-dose IL-2 immunotherapy and SARS-CoV-2 infection induce opposite transcriptional changes in immune cells. A Overview of the DILfrequency, COMBAT and INCOV cohorts. B Differential expression induced by low-dose IL-2 immunotherapy (DILfrequency) and SARS-CoV-2 infection (COMBAT and INCOV) on the top six up and downregulated genes from the anti-inflammatory gene expression signature induced by low-dose IL-2 treatment (IL2-AIS), previously identified in the DILfrequency cohort. Data shown depicts the log2 fold change (FC) values in each cohort and were calculated in the different immune subsets identified in the respective study. P values and additional IL2-AIS constituent genes are shown in Additional file 3: Fig. S1. C Distribution of the IL2-AIS scores in T cells from each participant group in the COMBAT cohort. Data was stratified by disease group and COVID-19 disease severity. P values were calculated by comparing each patient group with healthy controls using a two-sided Mann–Whitney U test followed by FDR adjustment. D Mean IL2-AIS scores in CD8+ T cells from each participant group in the INCOV cohort from samples collected during the first 14 days after symptoms onset. Data was stratified by COVID-19 disease severity. P values are calculated by comparing the severe or critical COVID-19 group with the mild COVID-19 group using a two-sided Mann–Whitney U test. In C and D, each box ranges from the first quartile (Q1) to the third quartile (Q3), with a central line indicating the median. Treg, regulatory T cells. Tconv, conventional T cells. MNP, mononuclear phagocytes. The IL2-AIS scores were derived from pseudo-bulk samples aggregated from single-cell RNAseq data of PBMCs
A recent research conducted by the University of Oxford-UK, highlights the discovery of a prolonged pro-inflammatory gene expression profile in individuals recovering from COVID-19. This transcriptional signature, characterized by an elevated immune response, may have far-reaching consequences on the health of post-acute COVID-19 patients, potentially contributing to autoimmune diseases, viral reactivation, and disruptions in the host immune system-microbiome ecosystem.
Unraveling the Long-Term Effects of COVID-19
As the pandemic continues to evolve, researchers have gained access to a wealth of data, including large single-cell transcriptomics datasets that shed light on the immune response to SARS-CoV-2 infection and its lasting impact on patients.
While the acute phase of COVID-19 has been extensively studied, less attention has been given to understanding the chronic consequences of the disease, often referred to as post-acute sequelae of COVID-19 (PASC). PASC is characterized by persistent symptoms that linger even after the initial infection has resolved as covered in various studies and past
rong>COVID-19 News reports. Understanding PASC is critical given the ongoing risk of reinfection with novel variants and the potential for further outbreaks.
Recent research, conducted using a targeted single-cell multiomics approach, identified an anti-inflammatory gene expression signature induced by low-dose interleukin-2 (IL-2) treatment in individuals with type 1 diabetes. Surprisingly, this signature persisted for an extended period, even after Treg (regulatory T cell) frequencies returned to baseline. This signature represented a shift toward an anti-inflammatory immune environment, suggesting that low-dose IL-2 treatment might have a therapeutic application in modulating immune responses.
Linking COVID-19 and the IL2-AIS Signature
Drawing connections between the IL2-AIS signature and COVID-19, researchers found that several genes associated with the IL2-AIS signature, such as CISH, AREG, DUSP2, NFKBIA, and TNFAIP3, displayed differential expression in COVID-19 patients. These findings prompted an investigation into whether COVID-19 induced a similar transcriptional profile. The results were striking.
Using single-cell transcriptomic data from the Oxford COVID-19 Multi-omics Blood Atlas (COMBAT) and the INCOV cohorts, researchers discovered a core set of co-regulated genes from the IL2-AIS signature that exhibited an inverted expression pattern in T, B, and NK cells, as well as monocytes of COVID-19 patients. Instead of promoting an anti-inflammatory environment, these genes indicated a pro-inflammatory response.
The Prolonged Pro-Inflammatory Signature in COVID-19 Patients
This pro-inflammatory signature was not short-lived. It persisted in COVID-19 patients for at least two months after the onset of clinical symptoms. Interestingly, this signature was not observed in response to influenza infection or sepsis, indicating its specificity to SARS-CoV-2. Gene network analysis pointed towards the central role of the transcription factor NF-κB in regulating these transcriptional alterations. NF-κB has previously been linked to severe COVID-19 cases.
Mechanisms Underlying Transcriptional Changes
Understanding the mechanisms underlying these transcriptional changes is crucial. During the acute phase of COVID-19, patients often exhibit higher levels of classical pro-inflammatory cytokines like IL-6, IFNa, and TNF. It is plausible that this pro-inflammatory environment during the acute phase could have a prolonged effect on the immune transcriptional profile. Pro-inflammatory cytokines can bind to the extracellular matrix, potentially maintaining higher local concentrations of cytokines at the infection site and immune tissues.
This prolonged exposure to cytokines may contribute to the transcriptional signature detected in this study. A prolonged pro-inflammatory environment has also been reported in mild COVID-19 patients, with alterations detected even 3–5 months post-infection.
Implications for Health and Long COVID-19
The persistence of this transcriptional alteration raises concerns about its health implications. While patients may be asymptomatic during this period, it could increase the risk of developing PASC, autoimmune diseases, or other health complications including new onsets of cancer. Moreover, individuals who experience repeated infections with SARS-CoV-2 may be at a heightened risk of suffering from the cumulative effects of this pro-inflammatory signature, further exacerbating the burden of post-acute sequelae.
Potential Therapeutic Applications
One intriguing aspect of this research is the potential therapeutic application of low-dose IL-2 immunotherapy to mitigate the prolonged immune dysfunction in post-acute COVID-19 patients and reduce the occurrence of PASC. IL-2, known to bind to the extracellular matrix, might promote a prolonged anti-inflammatory state, reducing the production and accumulation of pro-inflammatory cytokines in tissues. Low-dose IL-2 treatment has a strong safety record and has previously shown promise in reducing the incidence of infections in other patient populations. However, it's important to administer it in the convalescent phase, well after the acute inflammation has subsided, to avoid hyperactivation of the immune response during the acute phase of the disease.
Conclusion
In conclusion, the research conducted by the University of Oxford sheds light on the long-lasting effects of SARS-CoV-2 infection on the immune system. The discovery of a prolonged pro-inflammatory transcriptional signature in post-acute COVID-19 patients underscores the need for continued research into the mechanisms underlying this alteration and its potential health consequences.
Furthermore, the study suggests that low-dose IL-2 immunotherapy could hold promise as a therapeutic approach to mitigate immune dysfunction and reduce the risk of post-acute complications. As the pandemic evolves, understanding the long-term effects of COVID-19 remains crucial for the well-being of individuals and communities worldwide.
The study findings were published in the peer reviewed journal: Genome Medicine.
https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-023-01227-x
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
