Pathological Effects Of SARS-Cov-2 On The Cell Nucleus And Their Implications In Long COVID
COVID-19 News - SARS-Cov-2 - Cell Nucleus Apr 15, 2023 2 years, 5 days, 6 hours, 16 minutes ago
COVID-19 News: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic, which has caused significant morbidity and mortality worldwide. In addition to acute infection, long-term complications, known as Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), have become a major concern. The cytopathic effects of SARS-CoV-2 on cellular organelles, including the nucleus, have been observed, leading to morphological and functional alterations. This
COVID-19 News article aims to discuss these effects and their implications in Long COVID.
 SARS-CoV-2 Proteins And Nuclear Interactions
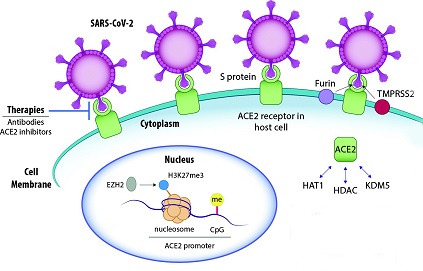
SARS-CoV-2 Proteins And Nuclear Interactions
Several SARS-CoV-2 proteins can interact with host cell nuclear components, leading to the suppression of the host's antiviral response. Some of these interactions involve the alteration of nuclear-cytoplasmic transport, impacting the host's interferon-mediated response. The viral proteins responsible for such alterations include the ORF6, nsp9 and nsp1 proteins.
ORF6 is known to interact with nuclear pore proteins, such as RAE1 and NUP98, causing an alteration in nuclear import. It can also reduce nuclear size and disrupt cell growth when overexpressed. ORF6, along with ORF3b, can block interferon (IFN) signaling by inhibiting the entry of transcription factors such as STAT into the nucleus, impairing the induction of transcription of IFN-stimulated genes (ISGs).
Similarly, the nsp9 protein of SARS-CoV-2 can interact with nuclear pore complex proteins, including NUP54, NUP58, NUP62, NUP88, and NUP214. SARS-CoV-2 N protein can also block the expression of ISGs by inhibiting the phosphorylation of STAT1 and STAT2, which is crucial for their translocation to the nucleus. The nsp1 protein inhibits the nuclear translocation of interferon regulatory factor 3 (IRF3), an essential transcription factor for antiviral responses.
Inhibition Of mRNA Export And Nuclear Morphological Changes
SARS-CoV-2 can inhibit mRNA export from the host nucleus, presumably through the binding of nsp1 to nuclear RNA export factor 1 (NXF1), thereby suppressing host cell gene expression. In addition to functional changes, morphological alterations have been observed in the nuclei of infected cells. Histopathological examinations of post-mortem tissues from COVID-19 patients revealed nuclear pleomorphism, vacuolization, and enlarged nuclei in various cell types, including salivary gland duct lining epithelium, acinar cells, keratinocytes of the gingival junctional epithelium, and type II pneumocytes.
Fusion between infected and healthy cells can lead to the formation of multinucleated syncytia, particularly in type II pneumocytes. These findings, along with the long-term persistence of viral RNA and the occurrence of thrombosis, are three of the main hallmarks of advanced COVID-19 disease.
Folate-Mediated Monocarbon Metabolism And Long COVID
Recent studies have reported that folate-mediated monocarbon metabolism (FOCM) may be altered in some patients with Long COVID. FOCM is a metabolic network occurring in the nucleus, mitochondria, and cytoplasm, which may be stressed during the viremia phase
of SARS-CoV-2 replicatio, leading to serine and glutathione depletion, increased oxidative stress, and altered methyl group delivery mechanisms. S-adenosylmethionine (SAMe), a co-substrate involved in methyl group transfer, plays a central role in this metabolic pathway and is transported to the cell nucleus through nuclear pores.
Interactions between SARS-CoV-2 and host cell nuclear pores can alter the nuclear import of certain molecules, potentially impacting the nuclear transport of SAMe and contributing to the development of FOCM in infected cells.
Moreover, the SARS-CoV-2 nsp9 protein is known to interact with methionine adenosyltransferase 2B (MAT2B), responsible for SAMe biosynthesis from methionine and ATP, which could further affect this metabolic pathway.
Persistence Of Viral Proteins And Implications In Long COVID
Consistent with cytopathic effects on other cellular organelles, the persistence of viral proteins within infected cells or side effects of their interactions with host proteins could also alter nuclear morphology and impair nuclear trafficking. This may result in an attenuated long-term antiviral response and altered gene expression. However, there is limited evidence for the possible implications of nuclear cytopathies caused by SARS-CoV-2 in the development of Long COVID disease.
As more research is conducted on the cytopathic effects of SARS-CoV-2 on the nucleus, our understanding of the mechanisms behind Long COVID will likely improve. This knowledge may help develop targeted therapies for patients suffering from Long COVID and contribute to the overall understanding of the pathogenesis of SARS-CoV-2 infection.
Conclusion
SARS-CoV-2 infection can lead to various cytopathic effects on the nucleus, including morphological and functional alterations. These effects may result from interactions between viral proteins and host cell nuclear components, leading to the suppression of the host's antiviral response, inhibition of mRNA export, and altered gene expression. Furthermore, the involvement of SARS-CoV-2 proteins in FOCM disruption may be a contributing factor to the development of Long COVID.
A better understanding of the cytopathic effects of SARS-CoV-2 on the nucleus and their implications in Long COVID could help guide future research and the development of targeted therapies for patients suffering from persistent post-infection symptoms. As the COVID-19 pandemic continues to evolve, expanding our knowledge of the virus and its long-term consequences remains a priority for improving patient outcomes and public health.
References:
SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation
https://pubmed.ncbi.nlm.nih.gov/33637958/
Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex
https://pubmed.ncbi.nlm.nih.gov/33360543/
SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling
https://pubmed.ncbi.nlm.nih.gov/33097660/
SARS-CoV-2 N protein antagonizes type I interferon signaling by suppressing phosphorylation and nuclear translocation of STAT1 and STAT2
https://pubmed.ncbi.nlm.nih.gov/32953130/
Nsp1 protein of SARS-CoV-2 disrupts the mRNA export machinery to inhibit host gene expression
https://pubmed.ncbi.nlm.nih.gov/33547084/
A SARS-CoV-2 protein interaction map reveals targets for drug repurposing
https://pubmed.ncbi.nlm.nih.gov/32353859/
Innate cellular response to virus particle entry requires IRF3 but not virus replication
https://pubmed.ncbi.nlm.nih.gov/14747536/
Salivary glands are a target for SARS-CoV-2: a source for saliva contamination
https://pubmed.ncbi.nlm.nih.gov/33834497/
Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis
https://pubmed.ncbi.nlm.nih.gov/33712574/
Syncytia formation by SARS-CoV-2-infected cells
https://pubmed.ncbi.nlm.nih.gov/33522642/
Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology
https://pubmed.ncbi.nlm.nih.gov/33158808/
Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer's Disease and Long COVID
https://pubmed.ncbi.nlm.nih.gov/35056324/
Cloning, expression, and functional characterization of the beta regulatory subunit of human methionine adenosyltransferase (MAT II)
https://pubmed.ncbi.nlm.nih.gov/10644686/
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/the-dynamic-interplay-between-sars-cov-2-and-mitochondrial-function-implications-for-long-covid
https://www.thailandmedical.news/news/the-interplay-between-sars-cov-2-and-the-endoplasmic-reticulum-and-the-resulting-covid-19-symptoms-and-long-covid-manifestations
