PIEZO1 Identified As Playing A Key Role In COVID-19-Associated Endothelial Dysfunction
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 05, 2024 1 year, 9 months, 3 weeks, 2 days, 21 hours, 25 minutes ago
COVID-19 News: The ongoing global battle against the COVID-19 pandemic has underscored the critical importance of understanding the intricate mechanisms underlying the virus-host interaction. Emerging evidence points towards endothelial dysfunction as a central player in the multiorgan injuries associated with COVID-19, necessitating the exploration of novel therapeutic strategies targeting endothelial cells. One such promising candidate is the mechanosensitive ion channel PIEZO1, highly expressed in the blood vessels of various tissues. This study review by researchers from Changhai Hospital, Naval Medical University, Shanghai-China covered in
COVID-19 News report, delves into the potential role of PIEZO1 in mitigating COVID-19-associated endothelial dysfunction, shedding light on its implications for therapeutic development.
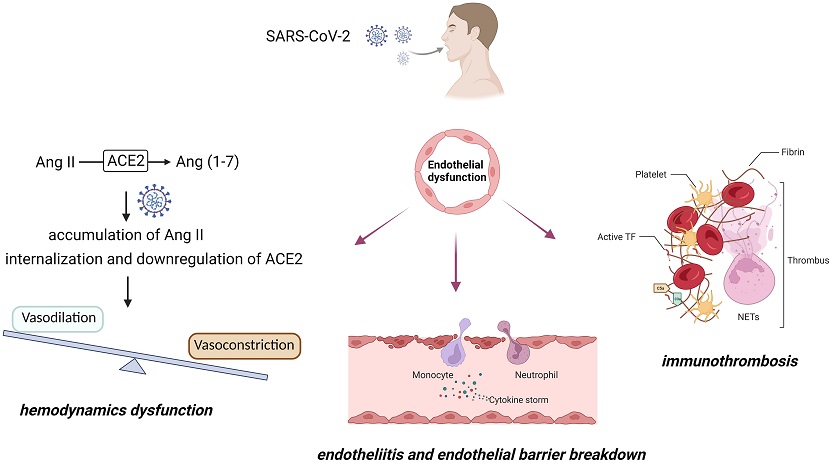 COVID-19-associated endothelial dysfunction
COVID-19-Associated Endothelial Dysfunction
COVID-19-associated endothelial dysfunction
COVID-19-Associated Endothelial Dysfunction
Beyond the respiratory symptoms commonly associated with COVID-19, extrapulmonary manifestations have drawn attention, with endothelial dysfunction and immunothrombosis emerging as key pathogenic mechanisms. The intricate interplay between SARS-CoV-2 infection, immune response activation, and inflammatory signaling leads to endothelial barrier disruption, coagulation cascade activation, and subsequent tissue damage. Cardiovascular complications have become a major concern, with hemodynamic dysfunction, endothelitis, vascular leakage, and thrombosis contributing to multiorgan damage.
Role of ACE2 and Angiotensin Signaling in COVID-19
ACE2, a critical enzyme in the cardiovascular system, serves as the main entry receptor for SARS-CoV-2. The virus's interaction with ACE2 leads to dysregulation of the renin-angiotensin system, resulting in elevated levels of Ang II. The accumulation of Ang II induces endothelial dysfunction, inflammation, and cardiovascular injury. Autoantibodies against Ang II, AT1, and ACE2 have been detected in COVID-19 patients, emphasizing the disturbance in vascular tone and the significance of targeting this pathway for therapeutic interventions.
Immunothrombosis and Endothelial Barrier Breakdown
SARS-CoV-2 infection directly impacts endothelial cells, leading to endotheliitis, vascular leakage, and tissue damage. The activation of NLRP3 inflammasome and release of pro-inflammatory cytokines contribute to increased vascular permeability. Immunothrombosis, involving the activation of coagulation cascade and formation of neutrophil extracellular traps (NETs), further exacerbates endothelial injury, leading to vascular occlusions.
Potential Roles of PIEZO1 in COVID-19
Endothelial PIEZO1, a mechanosensitive ion channel, plays a crucial role in maintaining vascular homeostasis. Its potential links to SARS-CoV-2-induced calcium disturbance highlight its involvement in the pathogenesis of COVID-19. PIEZO1's role in hemodynamic homeostasis, maintenance of the endothelial barrier, regulation of thrombosis, and modulation of ventilator-induc
ed lung injury suggests its multifaceted significance in COVID-19-associated complications.
Exploring the Potential of PIEZO1 in Specific Aspects of COVID-19:
-Calcium Disturbance: The interaction between SARS-CoV-2 and calcium channels, potentially involving PIEZO1, leads to physiological perturbations and endothelial damage. Inhibition of PIEZO1 may offer a therapeutic strategy for modulating calcium disturbance in COVID-19-associated endothelial dysfunction.
-Hemodynamic Homeostasis: PIEZO1, as a vital sensor of blood flow, regulates hemodynamic stability. Targeting PIEZO1 may preserve normal hemodynamics, offering a potential avenue for therapeutic interventions in COVID-19.
-Endothelial Barrier Maintenance: Autopsy studies indicate severe endothelial injury in COVID-19 patients. PIEZO1's role in angiogenesis and endothelial barrier function makes it a potential target for preserving pulmonary vascular integrity and preventing endothelial breakdown.
-Thrombosis Regulation: Dysregulation of PIEZO1 in conditions like type 2 diabetes leads to a prothrombotic response. Targeting PIEZO1 may offer a strategy for mitigating COVID-19-associated thrombosis.
-Ventilator-Induced Lung Injury: PIEZO1's involvement in mechanical stretching during ventilation highlights its role in VILI. Modulating PIEZO1 activity may alleviate lung injury, providing a potential therapeutic avenue for severe COVID-19 cases requiring mechanical ventilation.
-Sarcopenia: Severe sarcopenia observed in some COVID-19 patients may be linked to PIEZO1 downregulation. Activating endothelial PIEZO1 could potentially address COVID-19-associated sarcopenia.
-Long COVID: Endothelial dysfunction is a hallmark of long COVID. Targeting PIEZO1 may offer therapeutic promise for alleviating or preventing long COVID symptoms.
Challenges and Future Perspectives
Despite the promising potential of PIEZO1 as a therapeutic target, several challenges need to be addressed before its clinical application. The precise role of PIEZO1 in COVID-19 pathogenesis remains unclear, and off-target effects must be carefully examined. Ensuring the safety and efficacy of PIEZO1 modulators is crucial, considering its ubiquitous expression and diverse physiological functions. Further research should unravel the molecular mechanisms underlying PIEZO1-mediated endothelial dysfunction and validate its therapeutic potential through clinical trials.
Conclusion
In conclusion, understanding the role of mechanosensitive ion channels, particularly PIEZO1, in COVID-19-associated endothelial dysfunction presents a promising avenue for therapeutic intervention. The multifaceted functions of PIEZO1 in regulating vascular homeostasis, inflammation, endothelial barrier integrity, and thrombotic processes highlight its significance in the context of COVID-19 therapeutics. Future research should focus on unraveling the specific molecular mechanisms, conducting rigorous clinical trials, and exploring other mechanosensitive channels to provide a comprehensive understanding of the complex mechanotransduction pathways involved in COVID-19-associated endothelial dysfunction. Leveraging this knowledge may pave the way for the development of innovative therapeutic strategies to mitigate the impact of COVID-19 on endothelial function and improve patient outcomes.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1281263/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
