Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 03, 2024 5 months, 1 week, 2 days, 22 hours, 34 minutes ago
Medical News: In a groundbreaking study, scientists from Zhejiang Sci-Tech University, China, and the University of Toronto, Canada, have shed light on a specific protein modification, known as crotonylation, and its role in cancer progression. The study investigates how crotonylation - a modification process that adds a crotonyl group to proteins - impacts the development of various cancers. This discovery not only adds to our understanding of how cancers evolve but also suggests new possible pathways for developing targeted therapies.
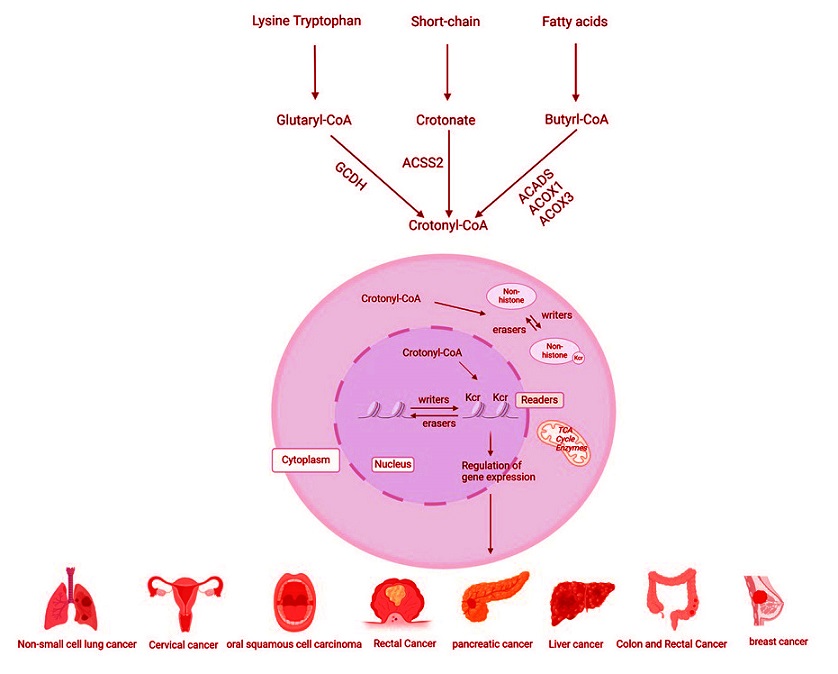 Regulatory mechanism of protein crotonylation. Catabolism of lysine or tryptophan generates crotonyl-CoA, catalyzed by GCDH. Circulating crotonate can be converted into crotonyl-CoA by ACSS2, and fatty acid β-oxidation produces crotonyl-CoA upon oxidation of butyryl-CoA, catalyzed by ACADS, ACOX1, or ACOX3. Protein crotonylation is affected by various writing, reading, and erasing proteins and is closely related to a variety of cancers
Regulatory mechanism of protein crotonylation. Catabolism of lysine or tryptophan generates crotonyl-CoA, catalyzed by GCDH. Circulating crotonate can be converted into crotonyl-CoA by ACSS2, and fatty acid β-oxidation produces crotonyl-CoA upon oxidation of butyryl-CoA, catalyzed by ACADS, ACOX1, or ACOX3. Protein crotonylation is affected by various writing, reading, and erasing proteins and is closely related to a variety of cancers
This
Medical News report reviews recent findings on the role of crotonylation in cancers like lung, cervical, liver, and others. These findings open doors to targeted cancer treatments based on understanding how proteins are modified in cells. Cancer is often described as uncontrolled cell growth, and protein modifications like crotonylation influence cell behavior. This modification is part of a process called post-translational modification (PTM), where proteins are altered to regulate cell functions like growth and division.
What is Crotonylation, and Why Does It Matter?
Post-translational modifications (PTMs) like crotonylation play a significant role in how our cells behave. Crotonylation was discovered relatively recently, in 2011, and involves attaching a crotonyl group - a small chemical group - to the amino acid lysine within proteins. This change can significantly affect how proteins function, influencing key processes like gene expression, DNA repair, and cellular metabolism.
One reason crotonylation is particularly exciting for cancer research is its unique structure. Crotonyl groups create a rigid, planar shape, differing from more flexible modifications like acetylation, which share some similar enzymes. This rigid structure potentially gives crotonylation the ability to alter protein behavior more strongly, affecting cells in unique ways.
The Role of Crotonylation in Cancer Development
In cancer, cells escape normal regulatory mechanisms and begin growing uncontrollably. Crotonylation plays an influential role here: it can change proteins in a way that may either prevent or promote cancerous behaviors in cells. By studying this process, scientists have identified various proteins affected by crotonylation, some of which are tied to tumor growth, survival, and metastasis. In particular, crotonylation is highly active in several types of cancers, including:
-Lung Cancer: Researchers found that crotonylation influences mitochondrial activity in lung cancer cells, aiding cancer cell survival.
-
Cervical Cancer:<
;br />
Crotonylation regulates the expression of a protein called HNRNPA1, linked to cell proliferation and tumor growth in cervical cancer.
-Prostate Cancer: The modification activates pathways that support tumor cell growth and spread, specifically affecting proteins that interact with androgen receptors, important in prostate cancer progression.
Key Proteins in Crotonylation: Writers, Readers, and Erasers
In cells, certain proteins are responsible for adding (writers), recognizing (readers), and removing (erasers) crotonyl groups. This study highlights that these “writer” enzymes are often responsible for the initial changes in proteins that drive cancerous growth.
-Writers: The protein P300 is one of the main writers, attaching crotonyl groups to histones and other proteins, potentially leading to tumor growth when it operates uncontrollably.
-Readers: Bromodomain and YEATS proteins are key “readers” that detect crotonylation, setting off further cellular responses that support cancer cell survival.
-Erasers: Histone deacetylases (HDACs) like HDAC1, HDAC2, and HDAC3 act as “erasers” by removing crotonyl groups from proteins, reversing these modifications.
The research highlights how manipulating these proteins could influence cancer progression, providing valuable insights into potential treatment approaches.
Lung Cancer and Mitochondrial Activity
Non-small cell lung cancer (NSCLC), a common and aggressive form of lung cancer, shows a high presence of crotonylation. Researchers observed that crotonylation in these lung cancer cells helped control mitochondrial autophagy - a cellular cleanup process that maintains healthy mitochondria, which power the cell.
In NSCLC, crotonylation increases the expression of a protein called BEX2, which interacts with proteins involved in autophagy. This connection between crotonylation and mitochondrial function may provide a new target for slowing the growth of NSCLC, as altering this crotonylation process could make cancer cells more vulnerable to treatment.
Cervical Cancer and the Role of HNRNPA1 Protein
Cervical cancer, one of the most common cancers affecting women, shows changes in the crotonylation process. Researchers noted that crotonylation increases the activity of a protein called HNRNPA1, which influences tumor cell growth, migration, and resistance to treatment. By altering HNRNPA1’s activity, crotonylation helps these cells evade normal growth controls.
Scientists have suggested that introducing external molecules that block crotonylation might help limit cervical cancer growth by regulating HNRNPA1 expression. This strategy offers a promising avenue for developing treatments that directly impact tumor development in cervical cancer.
Prostate Cancer and the Impact of Crotonylation on Androgen Receptors
Prostate cancer research in this study demonstrates that crotonylation significantly impacts androgen receptor signaling, a key pathway in prostate cancer progression. In prostate cancer cells, crotonylation is detected in proteins that help these cells grow and spread. This process specifically supports tumor cells' androgen receptor interactions, potentially offering a new target for treatments.
Bromodomain proteins like BRD4, which recognize crotonylated proteins, play an essential role in this process. By interacting with crotonylated histones, BRD4 can influence gene expression in ways that promote prostate cancer cell survival and proliferation.
Other Cancers Influenced by Crotonylation
The study further explored crotonylation’s role in other cancers, including pancreatic cancer, liver cancer, and oral squamous cell carcinoma. Each of these cancers showed a unique pattern of protein crotonylation affecting cancer cell behavior, underscoring the potential for crotonylation-targeted treatments across various cancer types.
For example, pancreatic cancer cells rely on enzymes altered by crotonylation to support the rapid growth typical of this aggressive cancer. By examining enzymes influenced by crotonylation, scientists identified potential points for intervention, where treatments might disrupt the metabolic processes supporting tumor growth.
Similarly, in hepatocellular carcinoma (HCC), a common type of liver cancer, crotonylation interacts with proteins involved in cell cycle control and cell migration, processes critical to cancer spread. Targeting these crotonylated proteins might slow the spread of HCC, providing a potential strategy to improve survival rates.
Conclusions: Crotonylation as a Future Target in Cancer Therapy
This research has broad implications for the future of cancer treatment. By understanding how crotonylation modifies proteins in ways that encourage cancer growth, scientists have identified several promising targets for new therapies. Interventions that adjust crotonylation levels, block its writers or readers, or amplify erasers could provide new ways to slow or stop cancer progression.
As researchers continue to investigate crotonylation, they are likely to discover additional details on how this modification supports cancer development across multiple cancer types. Given its widespread role in regulating proteins that drive tumor growth, crotonylation stands out as a potential target for therapeutic development in many cancers.
The study findings were published in the peer-reviewed journal: Cells.
https://www.mdpi.com/2073-4409/13/21/1812
For the latest Cancer News, keep logging on to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/study-finds-that-millennials-and-gen-x-are-at-higher-risk-for-17-types-of-cancer-unlike-generations-before-them
https://www.thailandmedical.news/news/formononetin-from-astragalus-provides-new-hope-against-multidrug-resistant-cancers
