Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 13, 2024 1 year, 6 days, 8 hours, 17 minutes ago
Medical News:
Influenza Research Highlights Cell Death Mechanisms
Influenza A virus (IAV) continues to be a formidable health challenge, causing seasonal epidemics and occasional pandemics. It is a segmented single-stranded RNA virus, primarily targeting respiratory tract epithelial cells. The body's immune system combats this virus using several mechanisms, including programmed cell death. Among these, pyroptosis, apoptosis, and necroptosis are significant players.
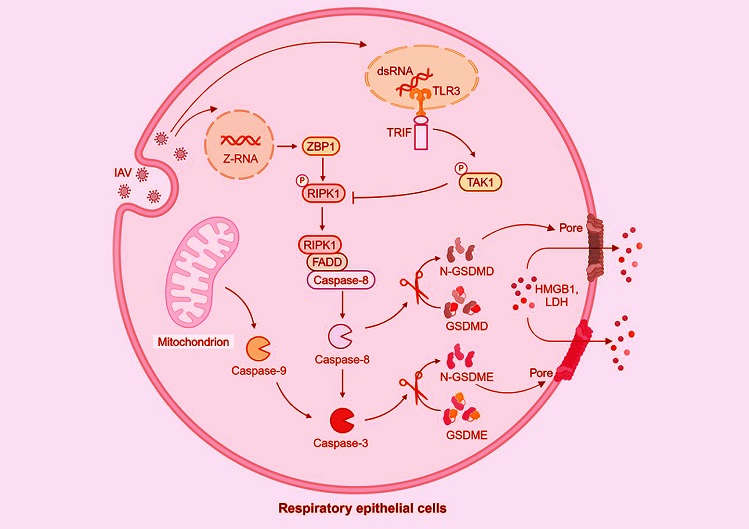 Graphical Abstract - Researchers Discover Role of TRIF and TAK1 in Influenza A Virus Pyroptosis
Graphical Abstract - Researchers Discover Role of TRIF and TAK1 in Influenza A Virus Pyroptosis
This
Medical News report delves into a recent study focusing on how IAV-induced pyroptosis functions and how cellular signaling pathways regulate this process. Researchers from Yangzhou University’s College of Veterinary Medicine, Jiangsu Co-innovation Center, and other affiliated institutions explored this phenomenon. The findings provide critical insights into how the virus interacts with respiratory cells and the body’s defense mechanisms.
What is Pyroptosis
Pyroptosis is a form of programmed cell death, distinct from apoptosis and necroptosis, playing a crucial role in the immune response. It involves the activation of gasdermin proteins, which create pores in the cell membrane, leading to inflammation and cell death. While this process helps alert the immune system, it can also be exploited by pathogens like IAV to enhance their replication.
IAV’s interaction with respiratory epithelial cells triggers pyroptosis by activating specific cellular proteins like caspases and gasdermins. The study identified the involvement of proteins such as GSDMD (gasdermin D) and GSDME (gasdermin E), which are activated by caspase-8 and caspase-3, respectively. These proteins form a cascade that leads to cell death, serving as both a defensive measure and a potential vulnerability.
Key Findings of the Study
Role of TRIF and TAK1 Pathways
One of the study's most intriguing discoveries was the role of TRIF (TIR domain-containing adaptor-inducing interferon-β) and TAK1 (transforming growth factor-beta-activated kinase 1) in regulating pyroptosis. TRIF, a protein involved in immune signaling, activates TAK1, which suppresses the activity of RIPK1, a kinase critical for pyroptosis.
When TAK1 is inhibited, as demonstrated using a specific inhibitor called 5Z-7-oxozeaenol (5Z), there is an enhancement in the activation of pyroptotic proteins like GSDMD and GSDME. This was further validated in mouse models, where TAK1 inhibition led to increased cell death in the lungs, highlighting the pathway's regulatory role.
Caspase Activation
The researchers confirmed that IAV-induced pyroptosis relies on caspase-8 and caspase-3. In knockout experiments, cells lacking these caspases exhibited reduced pyroptosis, underscoring their importance in the cell death process. Interestingly, the absence
of these proteins also led to enhanced virus replication, revealing a complex interplay between cell death and viral propagation.
Gasdermin Proteins and Viral Replication
Gasdermin proteins, especially GSDMD and GSDME, were shown to be essential for IAV-induced pyroptosis. Their activation was significantly reduced in cells where these proteins were knocked out. This not only inhibited pyroptosis but also increased viral replication, suggesting that while pyroptosis is destructive, it serves a protective role in limiting viral spread.
Insights from Mouse Models
In vivo studies reinforced the findings observed in cell cultures. Mice treated with the TAK1 inhibitor exhibited enhanced pyroptosis in their lung tissues, as evidenced by increased cleavage of caspase-8 and gasdermins. This points to TAK1’s crucial role in dampening excessive inflammation and controlling the extent of pyroptosis during infection.
Implications for Influenza Treatment
The study sheds light on the dual-edged nature of pyroptosis. While it helps control viral replication, excessive cell death can lead to severe inflammation, potentially resulting in complications like cytokine storms. Understanding the balance between these processes can guide therapeutic strategies. Targeting pathways like TAK1 or modulating caspase activity might offer novel approaches to mitigate the effects of severe influenza infections.
Conclusion
The study conducted by Yangzhou University and its affiliated institutions provides a comprehensive understanding of the mechanisms driving IAV-induced pyroptosis in respiratory epithelial cells. By delineating the roles of key proteins like TRIF, TAK1, and gasdermins, the research offers insights into potential therapeutic targets. These findings not only advance our knowledge of influenza pathogenesis but also pave the way for more effective treatments.
For the broader scientific community, the implications extend beyond influenza. Pyroptosis is a universal process observed in various infectious and inflammatory diseases. Thus, insights from this study could inform research into other conditions involving programmed cell death.
The study findings were published in the peer-reviewed journal: iScience.
https://www.sciencedirect.com/science/article/pii/S2589004224028086
For the latest on Influenza Research, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/influenza-mutations-and-drug-resistance-in-focus
https://www.thailandmedical.news/news/key-role-of-neil1-in-influenza-virus-proliferation-and-immune-response
https://www.thailandmedical.news/articles/influenza-or-flu
https://www.thailandmedical.news/articles/h5n1-avian-flu
