Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 25, 2024 1 year, 1 day, 11 hours, 50 minutes ago
COVID-19 News: Myocarditis, an inflammatory condition of the heart muscle, has gained significant attention as a complication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, causing the Coronavirus Disease 2019 (COVID-19). While COVID-19 primarily manifests as a respiratory illness, its impact on the cardiovascular system, particularly myocarditis, has raised concerns due to its potential for severe morbidity and mortality. Understanding the molecular mechanisms underlying SARS-CoV-2-induced myocarditis is crucial for developing effective therapeutic strategies. One emerging area of interest is RNA m5C methylation, a post-transcriptional modification implicated in various biological processes. This study review by researchers from Sichuan University-China, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital-China and West China Hospital-China that is covered in this
COVID-19 News report, aims to delve into the intricate interplay between RNA m5C methylation and SARS-CoV-2-induced myocarditis, shedding light on potential therapeutic avenues.
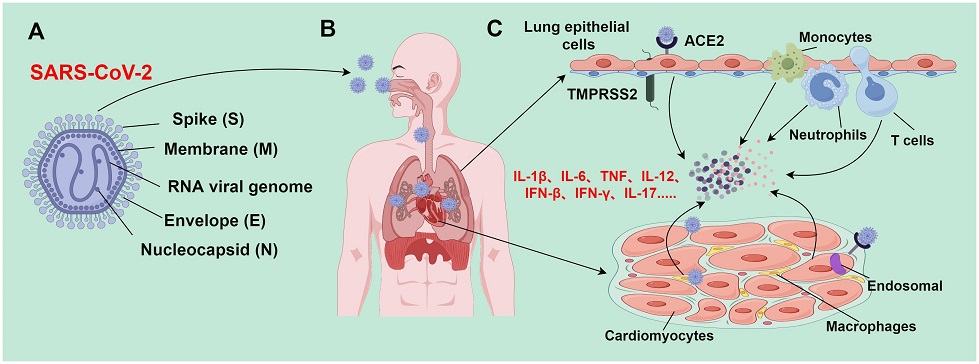 Mechanism of SARS-CoV-2-induced tissue damage. (A) SARS-CoV-2 is an enveloped, positive sense, and single-stranded RNA virus with a genome of about 30,000 nucleotides, which encodes major structural proteins such as spike protein (S), nucleocapsid protein (N), membrane protein (M), and envelope protein (E); (B) SARS-CoV-2 is transmitted to various body organs (such as the heart) mainly through the respiratory tract; (C) during epithelial infection, SARS-CoV-2 binds to ACE2 receptors; TMPRSS2 facilitates the virus to fuse with airway epithelial cells, leading to the release of viral genomic RNA into the cytoplasm. However, SARS-CoV-2 enters cardiomyocytes through endosomes-dependent mechanisms without requiring TMPRSS2. The SARS-CoV-2 infection process activates the immune system and triggers the release of a large number of inflammatory cytokines; these inflammatory cytokines subsequently recruit and activate more immune cells (such as monocytes, macrophages, neutrophils, and Th1 cells) to form a positive feedback loop. When the inflammation level reaches a certain threshold, inflammatory responses would be out of control, resulting in an inflammatory cascade. This inflammatory cascade can lead to inflammatory cell death (PANoptosis), and eventually trigger severe pathological changes in lung and heart tissues.
RNA m5C Methylation: An Overview
Mechanism of SARS-CoV-2-induced tissue damage. (A) SARS-CoV-2 is an enveloped, positive sense, and single-stranded RNA virus with a genome of about 30,000 nucleotides, which encodes major structural proteins such as spike protein (S), nucleocapsid protein (N), membrane protein (M), and envelope protein (E); (B) SARS-CoV-2 is transmitted to various body organs (such as the heart) mainly through the respiratory tract; (C) during epithelial infection, SARS-CoV-2 binds to ACE2 receptors; TMPRSS2 facilitates the virus to fuse with airway epithelial cells, leading to the release of viral genomic RNA into the cytoplasm. However, SARS-CoV-2 enters cardiomyocytes through endosomes-dependent mechanisms without requiring TMPRSS2. The SARS-CoV-2 infection process activates the immune system and triggers the release of a large number of inflammatory cytokines; these inflammatory cytokines subsequently recruit and activate more immune cells (such as monocytes, macrophages, neutrophils, and Th1 cells) to form a positive feedback loop. When the inflammation level reaches a certain threshold, inflammatory responses would be out of control, resulting in an inflammatory cascade. This inflammatory cascade can lead to inflammatory cell death (PANoptosis), and eventually trigger severe pathological changes in lung and heart tissues.
RNA m5C Methylation: An Overview
RNA m5C methylation, characterized by the addition of a methyl group to the fifth carbon atom of cytosine, is a prevalent modification observed in eukaryotic messenger RNAs (mRNAs), transfer RNAs (tRNAs), and ribosomal RNAs (rRNAs). This modification is catalyzed by RNA m5C methyltransferases, primarily members of the NOL1/NOP2/SUN domain (NSUN) family and DNMT2. Conversely, demethylation of m5C is facilitated by demethylases such as members of the Ten-eleven translocation (TET) protein family and ALKBH1.
Biological F
unctions of m5C Methylation
m5C methylation plays diverse roles in cellular processes, including RNA export, translation, stability maintenance, and cell cycle control. Notably, NSUN2, a prominent m5C methyltransferase, regulates m5C modification in various RNA species, influencing cellular functions ranging from tRNA structure maintenance to oxidative stress regulation. The intricate network of m5C methylation recognition proteins further modulates RNA stability, export, and immune response activation.
Molecular Mechanisms of SARS-CoV-2-Induced Myocarditis: An In-Depth Exploration
The pathogenesis of SARS-CoV-2-induced myocarditis involves a complex interplay of viral factors, host immune responses, and cellular mechanisms. Understanding these intricate molecular pathways is crucial for devising effective therapeutic strategies and mitigating the adverse cardiac effects of COVID-19.
-Viral Entry and Replication
SARS-CoV-2 gains entry into host cells primarily through the binding of its spike (S) protein to angiotensin-converting enzyme 2 (ACE2) receptors, which are abundantly expressed on the surface of cardiomyocytes and endothelial cells. This initial interaction facilitates viral fusion and internalization, allowing the viral RNA to enter the host cell cytoplasm. Once inside, the viral RNA serves as a template for viral RNA polymerase, leading to viral genome replication and the synthesis of viral proteins necessary for viral assembly and maturation.
-Inflammatory Activation and Myocardial Damage
Aside from direct cellular entry, SARS-CoV-2 can indirectly induce myocarditis through inflammatory activation mediated by various immune cells and signaling pathways. Extracellular vesicles released from infected cells, carrying viral RNA and proteins, can trigger inflammatory responses in neighboring cells and distant organs, including the heart. This phenomenon contributes to the recruitment of immune cells such as macrophages, T lymphocytes, and neutrophils to the cardiac tissue, leading to local inflammation and tissue damage.
-Respiratory Dysfunction and Hypoxia
One of the primary mechanisms contributing to myocardial damage during SARS-CoV-2 infection is respiratory dysfunction, particularly in severe cases requiring mechanical ventilation or intensive care support. Hypoxia, resulting from impaired lung function and gas exchange, leads to systemic hypoxemia and oxygen supply-demand imbalance in vital organs, including the heart. Prolonged hypoxia can exacerbate myocardial injury, impair cardiac function, and contribute to the development of myocarditis and heart failure.
-Cytokine Storms and Immune Dysregulation
A hallmark feature of severe COVID-19 is the dysregulated immune response characterized by the release of pro-inflammatory cytokines, often termed "cytokine storms." Excessive production of cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) can lead to systemic inflammation, endothelial dysfunction, and microvascular thrombosis. These immune-mediated processes contribute significantly to myocardial injury and dysfunction, further exacerbating the pathogenesis of SARS-CoV-2-induced myocarditis.
-ACE2/Angiotensin (1-7) Axis Dysregulation
The renin-angiotensin system (RAS), particularly the ACE2/angiotensin (1-7) axis, plays a crucial role in cardiovascular homeostasis and regulation of vascular tone. ACE2, besides serving as the cellular receptor for SARS-CoV-2, counteracts the effects of angiotensin II (Ang II) by converting it to angiotensin (1-7), which exhibits vasodilatory, anti-inflammatory, and anti-fibrotic properties. However, viral binding to ACE2 downregulates its expression and impairs its protective functions, leading to enhanced Ang II activity, vasoconstriction, inflammation, and oxidative stress in the myocardium. This dysregulation of the ACE2/Ang (1-7) axis contributes significantly to myocardial injury and the pathogenesis of COVID-19-related cardiovascular complications.
-Endothelial Dysfunction and Microvascular Thrombosis
SARS-CoV-2 infection is associated with endothelial dysfunction, characterized by impaired endothelial barrier function, increased vascular permeability, and aberrant release of vasoactive substances. Endothelial cell activation and damage promote platelet aggregation, coagulation cascade activation, and microvascular thrombosis, contributing to myocardial ischemia, infarction, and myocarditis. Additionally, viral-induced endothelial inflammation and dysfunction further exacerbate systemic vascular complications and multiorgan dysfunction seen in severe COVID-19 cases.
SARS-CoV-2-induced myocarditis
The molecular mechanisms underlying SARS-CoV-2-induced myocarditis are multifaceted, involving viral entry and replication, inflammatory activation, respiratory dysfunction, cytokine storms, ACE2/Ang (1-7) axis dysregulation, endothelial dysfunction, and microvascular thrombosis. These interconnected pathways contribute synergistically to myocardial injury, dysfunction, and the clinical spectrum of COVID-19-related cardiovascular complications. Targeting these molecular mechanisms through targeted therapies, immunomodulation, antiviral agents, and cardiovascular support strategies holds promise for improving outcomes and reducing the burden of myocarditis in COVID-19 patients.
Innate Immunity in SARS-CoV-2 Infection
The host innate immune response plays a pivotal role in combating viral infections, including SARS-CoV-2. Pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) detect viral components, triggering inflammatory signaling pathways and cytokine production. However, dysregulated immune responses, characterized by cytokine storms and TLR overactivation, can exacerbate myocardial injury in SARS-CoV-2-induced myocarditis.
Potential Role of m5C Methylation in Regulating SARS-CoV-2 Infection
Recent studies have highlighted the potential impact of m5C methylation on SARS-CoV-2 infection. NSUN2, a key m5C methyltransferase, exhibits decreased expression during SARS-CoV-2 infection, impacting viral replication and host immune responses. Moreover, m5C-modified RNA has been shown to modulate innate immune signaling pathways, potentially influencing the severity of SARS-CoV-2-induced myocarditis.
Challenges and Prospects
Despite advancements in understanding the role of m5C methylation in viral infections, several challenges remain. The identification and validation of specific m5C-modified sites on SARS-CoV-2 RNA require meticulous experimental scrutiny. Furthermore, elucidating the precise molecular mechanisms by which m5C methyltransferases and demethylases regulate host immune responses in SARS-CoV-2-induced myocarditis is imperative for developing targeted therapeutic interventions.
Conclusion
In conclusion, RNA m5C methylation emerges as a critical player in the pathogenesis of SARS-CoV-2-induced myocarditis. By modulating viral replication, immune response activation, and host cell gene expression, m5C methylation exerts multifaceted effects on the course and severity of myocardial injury during SARS-CoV-2 infection. Future research endeavors focusing on unraveling the intricate interplay between m5C methylation and host-virus interactions hold immense promise for advancing therapeutic strategies against SARS-CoV-2-induced myocarditis.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1380697/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
