SARS-CoV-2 Alters Host Cell Organelles While Reshaping Cellular Processes and Pathways
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 18, 2024 11 months, 2 weeks, 6 days, 13 hours, 23 minutes ago
Medical News: The Impact of SARS-CoV-2 on Our Cells
The SARS-CoV-2 virus, responsible for the COVID-19 pandemic, significantly changes how infected cells function. By hijacking cellular structures, particularly the endoplasmic reticulum (ER), the virus ensures its survival and replication.
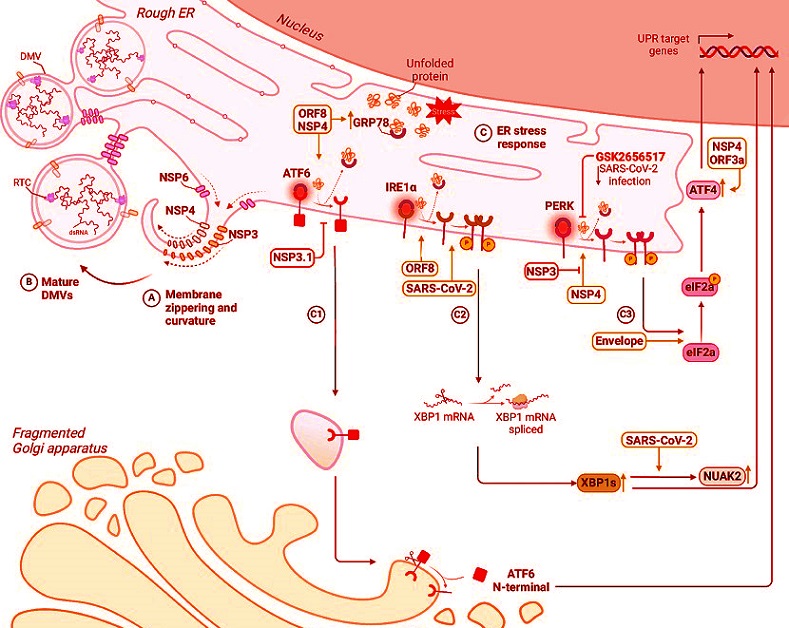 Functional and structural impact on ER upon SARS-CoV-2 infection
SARS-CoV-2 remodels the endoplasmic reticulum (ER) to facilitate viral replication. Non-structural proteins induce dramatic morphological changes in the ER, including the formation of double-membrane vesicles (DMVs), the site of viral genome replication. A, B Schematic representation of membrane curvature and zippering, leading to the formation of DMVs. C Model depicting the interplay between SARS-CoV-2-induced ER remodelling and UPR activation. The accumulation of misfolded proteins within the ER, a consequence of viral replication and ER stress, triggers the unfolded protein response (UPR). The three main UPR pathways ATF6 (C1), IRE1α (C2) and PERK (C3) are depicted with viral proteins that can directly or indirectly interfere with them.
Functional and structural impact on ER upon SARS-CoV-2 infection
SARS-CoV-2 remodels the endoplasmic reticulum (ER) to facilitate viral replication. Non-structural proteins induce dramatic morphological changes in the ER, including the formation of double-membrane vesicles (DMVs), the site of viral genome replication. A, B Schematic representation of membrane curvature and zippering, leading to the formation of DMVs. C Model depicting the interplay between SARS-CoV-2-induced ER remodelling and UPR activation. The accumulation of misfolded proteins within the ER, a consequence of viral replication and ER stress, triggers the unfolded protein response (UPR). The three main UPR pathways ATF6 (C1), IRE1α (C2) and PERK (C3) are depicted with viral proteins that can directly or indirectly interfere with them.
Scientists from the Telethon Institute of Genetics and Medicine in Italy, the University of Rome Tor Vergata, Scuola Superiore Meridionale in Naples, and the University of Campania Luigi Vanvitelli have meticulously examined these changes. Their findings uncover how the virus manipulates cellular pathways to thrive while disrupting natural defenses.
This
Medical News report focuses on three critical areas where the virus reshapes cellular functions: the remodeling of the endoplasmic reticulum, manipulation of autophagy (a recycling process in cells), and disruption of lipid metabolism. By understanding these effects, we gain insight into the battle between the virus and our cells.
How SARS-CoV-2 Alters the Endoplasmic Reticulum
The endoplasmic reticulum is a network of membranes within cells that produce and transport proteins and lipids. SARS-CoV-2 exploits this structure to create viral replication organelles (vROs) - safe havens where the virus replicates its genetic material. These vROs are formed using double-membrane vesicles (DMVs), which come from the ER’s membranes.
Key viral proteins, particularly NSP3, NSP4, and NSP6, play essential roles. NSP3 and NSP4 drive the formation of DMVs, while NSP6 helps curve and reshape ER membranes. Interestingly, a mutation in NSP6, found in several SARS-CoV-2 variants, enhances these functions, helping the virus evade immune detection and replicate more effectively.
The massive restructuring of the ER also triggers stress responses. Normally, cells activate pathways to restore balance when the ER is overwhelmed. However, SARS-CoV-2 manipulates this process to benefit its survival. For instance, the viral protein ORF8 activates specific stress pathways by binding to key proteins, such as GRP78, which support viral replication. This finding highlights how SARS-CoV-2 turns the cell's natu
ral defenses into tools for its growth.
Manipulation of Autophagy for Viral Benefit
Autophagy is the process by which cells recycle damaged components. It begins with the creation of membrane vesicles, called autophagosomes, that capture waste materials. These autophagosomes eventually fuse with lysosomes to break down their contents. For viruses like SARS-CoV-2, early stages of autophagy are beneficial because they provide membranes for replication structures.
The virus uses several proteins to manipulate autophagy. Proteins like ORF3a and ORF7a promote autophagosome formation but block their fusion with lysosomes. By halting this process, autophagosomes accumulate, and the virus prevents cellular waste degradation, allowing it to hijack resources.
ORF7a, for example, decreases SNAP29, a key protein needed for autophagosome-lysosome fusion.
The virus even triggers selective autophagy processes, such as mitophagy, which removes mitochondria - important energy-producing structures. ORF10 induces this process, leading to the breakdown of mitochondrial antiviral proteins (MAVS), further weakening the immune response.
Pharmacological approaches that restore autophagy or prevent its manipulation show promise in counteracting the virus. For example, certain compounds targeting ORF7a or ORF3a can reduce viral replication, offering hope for potential treatments.
How SARS-CoV-2 Alters Lipid Metabolism
Lipids are essential components of cellular membranes and energy storage. SARS-CoV-2 relies heavily on these molecules to build new virions and create replication structures. Infected cells show significant changes in lipid production, storage, and composition.
One notable change is the accumulation of ceramides, a type of lipid that helps the virus enter cells by facilitating interactions with ACE2 receptors. Drugs that block ceramide production, such as Fluoxetine and Maprotiline, have been shown to reduce viral entry, suggesting a potential therapeutic strategy.
SARS-CoV-2 also increases the production of lipids like phosphatidic acid (PA), which promotes membrane curvature - an essential step in forming replication organelles. Without PA, vRO formation is impaired, halting viral replication.
Lipid droplets (LDs), specialized storage compartments in cells, play a crucial role during infection. The viral protein NSP6 connects LDs to replication organelles, channeling stored lipids for viral growth. Over time, SARS-CoV-2 consumes these lipid stores, which are broken down through processes like lipolysis to release free fatty acids. Blocking lipolysis using specific drugs, such as Atglistatin, reduces viral replication, highlighting the importance of this pathway for the virus.
Study Conclusions
The study paints a clear picture of how SARS-CoV-2 rewires critical cellular pathways to support its life cycle. The virus transforms the ER into replication factories, manipulates autophagy to stall waste removal, and alters lipid metabolism to ensure a steady supply of essential building blocks. These findings reveal the virus's extraordinary ability to exploit cellular processes, turning cells into machines for its propagation.
Importantly, understanding these mechanisms provides scientists with valuable targets for treatment. By focusing on pathways like autophagy and lipid metabolism, future therapies could disrupt the virus's ability to survive without harming the host cell. The researchers emphasize that much remains to be explored, particularly the long-term impact of these changes on human health.
In conclusion, this study highlights the complex interplay between SARS-CoV-2 and host cells. The virus's ability to remodel cellular structures and processes ensures its survival while evading immune detection. Targeting these interactions could pave the way for new treatments, ultimately helping us better manage and control infections caused by SARS-CoV-2 and similar viruses.
The study findings were published in the peer reviewed journal: npj Viruses.
https://www.nature.com/articles/s44298-024-00076-8
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/university-of-california-study-finds-that-sars-cov-2-nsp4-protein-alters-endoplasmic-reticulum-structure
https://www.thailandmedical.news/news/new-insights-into-reticulophagy-and-its-role-in-viral-infections
https://www.thailandmedical.news/news/singapore-scientists-discover-that-sars-cov-2-spike-protein-undergoes-transformation-at-golgi-complex
https://www.thailandmedical.news/news/long-covid-and-the-hidden-changes-in-red-blood-cells
https://www.thailandmedical.news/news/pathological-effects-of-sars-cov-2-on-the-cell-nucleus-and-their-implications-in-long-covid
https://www.thailandmedical.news/articles/coronavirus
