SARS-CoV-2 Can Also Use Metalloproteinases, Specifically MMP-2 and MMP-9, For Viral Entry According To Recently Published Research
SARS-CoV-2 Research - Metalloproteinases, Specifically MMP-2 and MMP-9, For Viral Entry Mar 19, 2023 2 years, 4 weeks, 2 days, 16 hours, 11 minutes ago
SARS-CoV-2 Research: Scientists from University of Ottawa-Canada, Western University-Canada and University of Western Ontario-Canada have in December 2022 found that SARS-CoV-2 can also use Metalloproteinases, specifically MMP-2 and MMP-9, for viral entry and also for cell fusion.
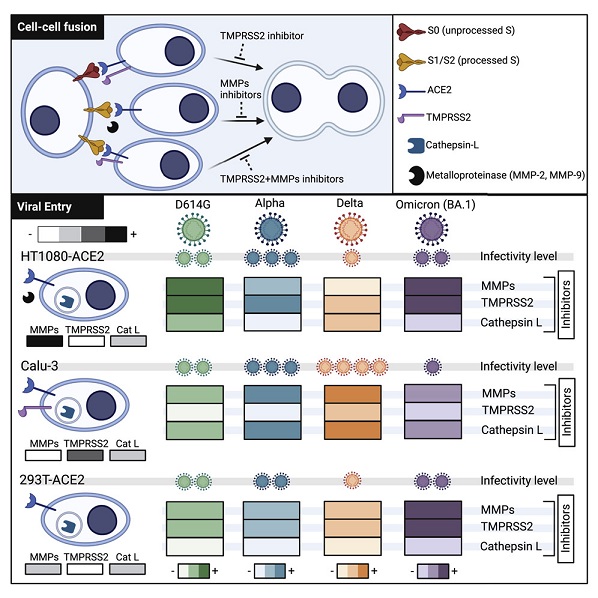 Graphical Abstract
Graphical Abstract
Typically, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike glycoprotein (S) binds to angiotensin-converting enzyme 2 (ACE2) to mediate membrane fusion via two distinct pathways: 1) a surface, serine protease-dependent or 2) an endosomal, cysteine protease-dependent pathway.
The
SARS-CoV-2 Research team however found in this new research that SARS-CoV-2 S has a wider protease usage and can also be activated by TMPRSS13 and matrix metalloproteinases (MMPs).
The study findings showed that MMP-2 and MMP-9 played roles in SARS-CoV-2 S cell-cell fusion and TMPRSS2- and cathepsin-independent viral entry in cells expressing high MMP levels. MMP-dependent viral entry required cleavage at the S1/S2 junction in viral producer cells, and differential processing of variants of concern S dictated its usage; the efficiently processed Delta S preferred metalloproteinase-dependent entry when available, and less processed Omicron S was unable to use metalloproteinases for entry. As MMP-2/9 are released during inflammation, they may play roles in S-mediated cytopathic effects, tropism, and disease outcome.
The key highlights of the study are:
-MMP-2 and MMP-9 enable SARS-CoV-2 S-mediated syncytia in the absence of TMPRSS2
-SARS-CoV-2 can enter cells via MMPs in a TMPRSS2- and cathepsin-independent manner
-MMP-dependent S activation requires prior S1/S2 processing
- Delta S can readily use MMPs for entry while Omicron S cannot. (However, this point has been disputed by a new study published in late January 2023 that shows the Omicron BA.1 variant is able to utilize MMPs for viral entry.
https://www.science.org/doi/10.1126/sciadv.add3867 )
Corresponding author, says Dr. Marceline Côté, a faculty associate professor University of Ottawa who is also the holder of the Canada Research Chair in Molecular Virology and Antiviral Therapeutics commented, "SARS-CoV-2 may be able to use proteins, which are typically secreted by some activated immune cells, to cause more damage and potentially infect a wider range of cells and tissues. The entry mechanism could also play a role in disease progression. The study findings also suggests that variants that gravitate toward metalloproteinases may cause more havoc.”
Dr. Côté added that the findings could have implications in the progression to severe illness and some post COVID-19 conditions, such as the complex array of post-infection symptoms known as &am
p;quot;long COVID."
The study findings were published in the peer reviewed journal: iScience.
https://www.cell.com/iscience/fulltext/S2589-0042(22)01588-7
A recent Bulgarian study published in February 2023 has also validated that SARS-CoV-2 can also use metalloproteinases, specifically MMP-2 and MMP-9, for viral entry, often with the consequences of increased risk of disease severity.
https://www.tandfonline.com/doi/full/10.1080/13102818.2023.2186137
Past studies have shown that SARS-CoV-1 and SARS-CoV-2 can enter cells via two distinct ACE2-dependent pathways: a surface serine protease pathway and an endosomal cathepsin protease pathway.
https://pubmed.ncbi.nlm.nih.gov/32142651/
https://pubmed.ncbi.nlm.nih.gov/32376634/
The study team show that the SARS-CoV-2 wild-type and variant of concerns (VOCs) S glycoprotein can also be triggered via metalloproteinases, specifically MMP-2 and MMP-9, for viral entry and cell-cell fusion. The ability to use this entry pathway required high expression of these proteases and proteolytic processing at the S1/S2 boundary in viral producer cells.
Accordingly, usage of this pathway by SARS-CoV-2 variants correlated with differential extents of S processing in viral producer cells; the efficiently processed S of Delta preferentially entered via the metalloproteinase route when available, while S of Omicron was mostly unprocessed and could not be activated via the metalloproteinase pathway. Given that metalloproteinases such as MMP-2/9 are released and highly expressed in the context of lung damage and inflammation during severe COVID-19, this mechanism of activation could play critical roles in S-mediated cytopathic effects, tropism, and overall pathogenesis.
With previous work reporting the presence of syncytia in the lung of deceased patients with COVID-19, the ability of SARS-CoV-2 S to mediate cell-to-cell fusion has been hypothesized to play roles in both pathogenesis and virus cell-to-cell propagation.
https://pubmed.ncbi.nlm.nih.gov/32221306/
https://pubmed.ncbi.nlm.nih.gov/33158808/
https://www.pnas.org/doi/abs/10.1073/pnas.2111400119
The formation of such multinucleated cells is believed to occur by the activation of SARS-CoV-2 S expressed at the cell surface of infected cells and fusion with neighboring cells expressing ACE2 and surface serine proteases such as TMPRSS2. However, the relatively low abundance of ACE2+ and TMPRSS2+ cells in the lower airways suggests that there may be additional factors able to activate S.
TMPRSS2-independent SARS-CoV-2 S-mediated cell-cell fusion has been previously observed in vitro and reported by other studies.
https://pubmed.ncbi.nlm.nih.gov/33310888/
https://pubmed.ncbi.nlm.nih.gov/33608407/
The study findings showed that metalloproteinases, including MMP-2 and MMP-9, are critical factors in SARS-CoV-2 S serine protease-independent fusion.
More precisely, the knockdown of MMP-2 and MMP-9 reduced S-mediated syncytia formation in the absence of serine proteases.
In addition, Alpha infection and replication in cells expressing high levels of MMP-2 and MMP-9 led to the substantial fusion of infected cells that were abrogated by broad-spectrum MMP inhibitors. Several studies have demonstrated increased levels of MMP-2 and MMP-9 in patients with severe COVID-19, which is associated with disease outcomes.
https://pubmed.ncbi.nlm.nih.gov/34449310/
https://pubmed.ncbi.nlm.nih.gov/35075175/
https://pubmed.ncbi.nlm.nih.gov/33232303/
In addition, infiltrating and activated neutrophils, which are potent sources of released MMP-9, are both associated with severe COVID-19.
https://pubmed.ncbi.nlm.nih.gov/33635335/
https://pubmed.ncbi.nlm.nih.gov/31986264/
https://pubmed.ncbi.nlm.nih.gov/32320677/
The study findings suggest a model by which inflammation and MMP production during COVID-19 could exacerbate virus-induced cytopathic effects further contributing to disease.
The study team demonstrated that entry of SARS-CoV-2 lentiviral particles, virus-like particles, and authentic Alpha variant into host cells can occur in a metalloproteinase-dependent manner.
Interestingly, this previously unrecognized entry pathway depended on S1/S2 processing in the viral producer cells. Therefore, this additional entry route is specific to SARS-CoV-2 and cannot be used by SARS-CoV-1, as SARS-CoV-1 S does not contain the critical S1/S2 furin cleavage site.
However, whether this additional entry pathway unique to SARS-CoV-2 played a role in its high transmissibility remain to be determined.
So far, multiple variants of SARS-CoV-2 have emerged since the beginning of the COVID-19 pandemic, with each having its own sets of mutations that enhance immune escape and/or transmissibility. Interestingly, variants such as Alpha, Delta, Kappa, and more recently Omicron possess mutations within the S1/S2 furin cleavage site. Notably, Delta S harbors the P681R mutation which improves S1/S2 processing and fitness over that of the ancestral virus and Alpha, which possesses the P681H mutation.
https://pubmed.ncbi.nlm.nih.gov/34614392/
https://pubmed.ncbi.nlm.nih.gov/34461056/
https://pubmed.ncbi.nlm.nih.gov/34823256/
https://pubmed.ncbi.nlm.nih.gov/34488225/
Past studies, including this study, have shown that Omicron S is less efficiently processed at the S1/S2 junction compared to ancestral S, S with a D614G mutation, and those of VOCs such as Delta.
https://pubmed.ncbi.nlm.nih.gov/35145066/
https://www.sciencedirect.com/science/article/pii/S221112472200701X
Hence, as expected, the study team found that Omicron S mediated reduced metalloproteinase-dependent cell-cell fusion.
Furthermore, VLPs expressing Omicron S were only slightly sensitive to metalloproteinase-dependent entry. Omicron S has three mutations near the furin cleavage site, H655Y, N679K, and P681H; however, the mechanism by which these or other mutations alter S processing remains to be determined.
Interestingly, the study team also found that an increase in Omicron S processing is not sufficient for efficient usage of the metalloproteinase-dependent entry pathway; suggesting that mutations in Omicron S2 likely affect recognition and/or cleavage by MMPs.
In addition, whether the inefficient use of the metalloproteinase pathway for the activation of S to mediate viral entry and cell-cell fusion plays a role in the apparent distinct clinical manifestations and tropism of Omicron is unclear.
The study findings of an additional entry pathway suggest a potential for increased tropism in the presence of MMPs during inflammation to cells that do not express serine proteases and could play important roles in dissemination and disease severity.
Another study recently reported a SARS-CoV-2 S-mediated metalloproteinase entry pathway in which ADAM10 was partially involved in different cell lines.
https://pubmed.ncbi.nlm.nih.gov/35708281/
Though the current study did not directly research a role for ADAM10 and it is still unknown if ADAM10 can cleave SARS-CoV-2 S, the current study findings and the past study above highlight the promiscuity of SARS-CoV-2 for host protease activation of S and intensifies the hurdles in the usage of host protease inhibitors for therapeutic purposes.
Past studies on the mouse hepatitis virus (MHV), another betacoronavirus, have reported a metalloproteinase-dependent cell-cell fusion and viral entry mechanisms, suggesting that metalloproteinases can activate multiple coronavirus S. Interestingly, unlike S activation mediated via cathepsins or surface serine proteases, the S activation triggered via metalloproteinases required prior S processing at the S1/S2 junction.
https://journals.asm.org/doi/full/10.1128/JVI.01564-16
Though more work is needed to determine whether MMP-2, MMP-9, and other metalloproteinases directly cleave S, the requirement for a processed S suggest that metalloproteinases can only cleave at the S2′ site. Most MMPs can cleave substrates with PXXXHy motifs in which the hydrophobic residue is often a leucine, but MMP-2 and MMP-9 can also cleave other groups of substrates.
A past study showed that MMP-9 has a distinct preference for Arg at both P(2) and P(1) which could represent a good match to the PSKR|S sequence of SARS-CoV-2 S2' site.
https://pubmed.ncbi.nlm.nih.gov/11279151/
Future studies are required to characterize the specific roles played by the metalloproteinases and to determine the specific S cleavage site involved in the metalloproteinase-dependent entry pathway.
Also, past studies investigating immune signatures of severe COVID-19 unveiled vascular endothelial growth factor A (VEGF-A), a disintegrin and metalloproteinases (ADAMs), and matrix metalloproteinases (MMPs) as potential markers for severe disease progression.
https://pubmed.ncbi.nlm.nih.gov/34698500/
https://pubmed.ncbi.nlm.nih.gov/33800947/
The study findings indicate that increased the secretion of MMPs during severe COVID-19 could exacerbate S-mediated cytopathic effects such as syncytia formation. Furthermore, it could also expand tropism by allowing entry in serine protease deficient cells, and potentially even in ACE2 deficient cells promoted by shed ACE2 induced by ADAM17 activity. Therefore, usage of the metalloproteinase pathway by current and future circulating SARS-CoV-2 variants could have profound implications for disease severity, outcome, and potential sequelae following recovery. Targeting MMPs, serine proteases, and cathepsins may be useful to reduce SARS-CoV-2 infection and COVID-19 severity.
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.
