SARS-CoV-2 Envelope Protein Down-Regulates Tight Junctional Proteins, Compromising The Integrity Of The Airway Epithelial Barrier
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 26, 2024 1 year, 10 months, 4 weeks, 2 days, 4 hours, 18 minutes ago
COVID-19 News: The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the subsequent coronavirus disease 2019 (COVID-19) pandemic have significantly impacted global health. A critical aspect of SARS-CoV-2 infection is its ability to disrupt the airway epithelial barrier, leading to heightened inflammation in the respiratory tract. This disruption contributes to respiratory symptoms and, in severe cases, can progress to acute respiratory failure. Understanding the molecular mechanisms underlying these processes is essential for developing targeted therapeutic interventions.
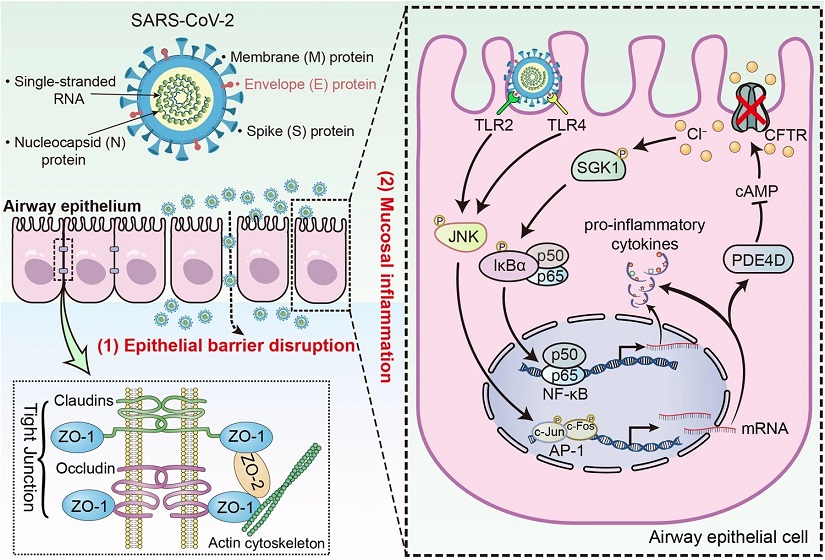 SARS-CoV-2 envelope protein impairs airway epithelial barrier function and exacerbates airway inflammation via increased intracellular Cl− concentration. Schematic diagram showing the pathophysiological role of SARS-CoV-2 envelope (E) protein in airway epithelial cells. SARS-CoV-2 E protein down-regulated tight junctional proteins, impairing airway epithelial integrity. E protein also activated TLR2/4-JNK-AP-1 signaling, leading to increased PDE4 expression and reduced cAMP levels. This disruption of cAMP signaling impaired CFTR-mediated Cl− transport, resulting in elevated [Cl−]i and activation of SGK1, which contributes to the ongoing inflammation in the airway epithelium
SARS-CoV-2 envelope protein impairs airway epithelial barrier function and exacerbates airway inflammation via increased intracellular Cl− concentration. Schematic diagram showing the pathophysiological role of SARS-CoV-2 envelope (E) protein in airway epithelial cells. SARS-CoV-2 E protein down-regulated tight junctional proteins, impairing airway epithelial integrity. E protein also activated TLR2/4-JNK-AP-1 signaling, leading to increased PDE4 expression and reduced cAMP levels. This disruption of cAMP signaling impaired CFTR-mediated Cl− transport, resulting in elevated [Cl−]i and activation of SGK1, which contributes to the ongoing inflammation in the airway epithelium
SARS-CoV-2 is an enveloped virus with a positive-sense single-stranded RNA genome. Among its structural proteins are the Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N) proteins. While much attention has been focused on the S protein's role in viral entry and fusion, the contributions of the E protein to pathogenesis are less understood. Recent studies have suggested that the E protein may play a significant role in disrupting the airway epithelial barrier and exacerbating inflammation in the respiratory tract. This
COVID-19 News article delves into a study by researchers from The First Affiliated Hospital of Guangzhou Medical University, Guangzhou-China AND Sun Yat-sen University, Guangzhou-China that explores the mechanisms by which the SARS-CoV-2 E protein influences airway epithelial integrity and inflammatory responses.
Role of Tight Junctions in Airway Epithelial Barrier Function
The airway epithelial barrier serves as the first line of defense against respiratory pathogens. It comprises tight junctions, mucociliary clearance mechanisms, and immune cells. Tight junctions are critical components of this barrier, composed of proteins such as claudins, occludin, and zona occludens (ZO) proteins. These proteins maintain cell-cell adhesion and regulate paracellular permeability, thereby preventing pathogens from breaching the epithelial layer.
During respiratory virus infections, including SARS-CoV-2, the expression and organization of tight junction proteins can be dysregulated, leading to compromised barrier function. Studies have shown that high viral loads of SARS-CoV-2 can disr
upt airway epithelial tight junctions, facilitating the entry of pathogens into the underlying tissue. The interaction between SARS-CoV-2 E protein and tight junction proteins like ZO-1 has been implicated in this process, highlighting the crucial role of E protein in triggering epithelial barrier dysfunction.
Intracellular Chloride (Cl−) Homeostasis and Signaling in Airway Epithelial Cells
Intracellular chloride (Cl−) concentration ([Cl−]i) is tightly regulated in cells and plays diverse roles in cellular functions, including signaling and inflammation. Disruption of intracellular Cl− homeostasis has been associated with inflammatory disorders. The cystic fibrosis transmembrane conductance regulator (CFTR), a chloride channel, is essential for maintaining Cl− transport across epithelial cells.
Studies have shown that respiratory pathogens, including SARS-CoV-2, can impact CFTR function, leading to imbalances in [Cl−]i. The E protein of SARS-CoV-2 has been implicated in modulating Cl− transport and intracellular signaling pathways. Specifically, E protein stimulation has been associated with increased [Cl−]i through the upregulation of phosphodiesterase 4D (PDE4D) expression via Toll-like receptor (TLR) signaling and c-Jun N-terminal kinase (JNK) activation. Elevated [Cl−]i can further contribute to heightened inflammation in the airway epithelium.
Inflammatory Signaling Pathways Activated by SARS-CoV-2 E Protein
The inflammatory response in airway epithelial cells following SARS-CoV-2 infection involves complex signaling pathways. Toll-like receptors (TLRs), particularly TLR2 and TLR4, play key roles in recognizing viral components and initiating immune responses. Activation of TLR2/4 by SARS-CoV-2 E protein triggers downstream signaling cascades, including the JNK pathway and activator protein-1 (AP-1) activation.
The JNK signaling pathway is crucial in mediating inflammatory responses and has been implicated in various viral infections. SARS-CoV-2 E protein-induced activation of JNK leads to increased expression of pro-inflammatory cytokines and chemokines, contributing to airway inflammation. Additionally, the JNK-AP-1 pathway mediates the inflammatory effects of E protein, highlighting its role as a key modulator of airway inflammation during SARS-CoV-2 infection.
Role of Serum/Glucocorticoid Regulated Kinase 1 (SGK1) in Airway Inflammation
Serum/glucocorticoid regulated kinase 1 (SGK1) is a serine/threonine kinase implicated in modulating inflammatory responses and NF-κB activation. SGK1 has been identified as a Cl−-sensitive kinase, with higher Cl− levels correlating with increased SGK1 activity. SARS-CoV-2 E protein stimulation has been shown to induce SGK1 phosphorylation, leading to NF-κB activation and pro-inflammatory cytokine production.
Inhibition of SGK1 has demonstrated anti-inflammatory effects in airway epithelial cells exposed to SARS-CoV-2 E protein, suggesting SGK1 as a potential therapeutic target for mitigating airway inflammation in COVID-19. Targeting the Cl−-SGK1 signaling axis may offer novel strategies for managing inflammatory lung conditions associated with viral infections.
Potential Therapeutic Interventions and Future Directions
Understanding the intricate interplay between SARS-CoV-2 E protein, intracellular Cl− dynamics, and inflammatory signaling pathways provides insights into potential therapeutic interventions. Targeting CFTR function, modulating Cl− transport, and inhibiting downstream inflammatory mediators such as SGK1 and JNK represent promising avenues for developing targeted therapies against COVID-19-associated airway inflammation.
Pharmacological agents that regulate Cl− homeostasis, such as PDE4 inhibitors and CFTR modulators, show potential in mitigating intracellular Cl− accumulation and dampening inflammatory responses induced by SARS-CoV-2 E protein. Further research into the specific mechanisms underlying these therapeutic targets and their efficacy in preclinical and clinical settings is warranted.
In conclusion, the SARS-CoV-2 E protein plays a multifaceted role in disrupting airway epithelial integrity, modulating Cl− homeostasis, and driving inflammatory responses in the respiratory tract. Elucidating these mechanisms not only enhances our understanding of COVID-19 pathogenesis but also guides the development of targeted therapies aimed at preserving airway barrier function and mitigating excessive inflammation in the lungs. Continued research in this field is essential for advancing our strategies against respiratory viral infections and related inflammatory lung diseases.
The study findings were published in the peer reviewed journal: Signal Transduction and Targeted Therapy (Springer Link).
https://link.springer.com/article/10.1038/s41392-024-01753-z
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
