SARS-CoV-2 News: European And Chinese Researchers Provide More Evidence That Pangolins Are Not The Intermediates In Transmission Of SARS-CoV-2 To Humans
Source: SARS-CoV-2 News Oct 05, 2020 4 years, 6 months, 3 weeks, 17 hours, 4 minutes ago
SARS-CoV-2 News: Researchers from the University of Barcelona-Spain; Xiamen University0China; and IHU-Méditerranée Infection and CNRS-France provide more new evidence to dispute the hypothesis that pangolins were the intermediate animals in SARS-CoV-2 transmission to humans.

Their research findings are published in the peer-reviewed journal:
Infection, Genetics and Evolution https://www.sciencedirect.com/science/article/pii/S1567134820303245
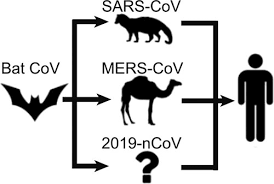
There have been numerous studies focused on identifying the intermediate animal that is potentially involved in the transmission of the SARS-CoV-2 virus to humans.
Certain past studies assumed that the pangolin was the intermediary between the virus and humans based on the presence of virus related to SARS-CoV-2 in Malayan pangolins, ACE2 receptor polymorphism, and the similarities in sequence between the receptor-binding domain (RBD) of pangolin and human Sarbecoviruses.
New emerging studies have reported that the binding affinity of the pangolin ACE2 receptor for the RBD of the virus is low.
The study team proposed a different model named the ‘circulation model’ to illustrate how SARS-CoV-2-related coronaviruses could have circulated in different species, including humans, before COVID-19 emerged.
Although many past studies indicated that bats and pangolins were the culprits in COVID-19 transmission to humans, none of the studies clarified the actual role of these animals in the emergence of the COVID-19 pandemic.
The SARS-CoV-2 virus is suspected of having emerged from bats and was said to be closely related to the Sarbecoviruses MN996532_raTG13 and RmYN02 from the Chinese horseshoe bats
Rhinolophus affinis and
Rhinolophus malayanus, respectively.
The new findings of this research did not agree with the currently proposed spillover model for zoonotic emergence.
Furthermore, the in-depth genomic analysis failed to support recombination in SARS-CoV-2 and showed that it has been circulating in bats for several decades now. Moreover, the drawback of metagenomic analyses that some previous studies performed is that there is not enough evidence to show that different parts come from the same virus and the recombinants came from artifactual assembly mosaics.
It is known that the SARS-CoV-2 spike protein S1 binds to the human ACE2 molecules on the cell surface. The detailed analysis of ACE2 3D structures indicated that the amino acids found in the 30–41, 82–93, and 353–358 regions play a key role in interactions with the viral S1 spike protein.
This set off a series of in silico analyses of ACE2 polymorphism that aimed at predicting which putative intermediate host animal might bind best to the ACE2 receptor so as to capture a SARS-CoV-2-like virus that is transmissible to humans.
Interestingly oth
er than pangolin, the novel coronavirus was shown to bind to the ACE2 receptor from a host of animals, including Chinese horseshoe bats, cats, civets, turtles, monkeys, ferrets, dogs, Chinese hamsters, cows, buffaloes, sheep, swine, and pigeons.
The study team says that the SARS-CoV-2 coronavirus does not preadapt to the host; instead, there is a host-driven selection of viruses post-exposure that can evade immune surveillance. The virus is already present in humans or animal species close to humans. A random event like a mutation suddenly makes it pathogenic or more invasive.
The conclusion coming from these data is, in agreement with a previous study that the pangolin is not the intermediate species in the transmission of SARS-CoV-2 from bats to humans. It appears to only be a parallel host infected by a Sarbecovirus very closely related to SARS-CoV-2 with whom it shares a common ancestor.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1008421
Furthermore, the COVID-19 epidemic did not start in December 2019 in the Huanan Seafood Wholesale Market (HSWM) where pangolins were supposed to be sold but earlier and outside HSWM
https://www.frontiersin.org/articles/10.3389/fmed.2020.00223/full?utm_term=RWRpdG9yaWFsX0Nvcm9uYXZpcnVzVGhlV2Vla0V4cGxhaW5lZC0yMDA1MDg%3D&utm_source=esp&utm_medium=Email&utm_campaign=CoronavirusTheWeekExplained&CMP=coronavirusweek_email
Searching for a culpirt in the wild is not realistic according to the researchers.
According to the circulation model there is a broad circulation of viruses in different species, including humans, upon contact but with no epidemic to follow. This fits with the observation that humans have been a lot more exposed to various viruses than expected and without any related epidemic
https://academic.oup.com/cid/article/50/12/1636/305066
Out of 60 viruses involved in zoonoses, 59 are RNA viruses.
https://www.sciencedirect.com/science/article/abs/pii/S0966842X14002480
Several RNA viruses, among which the Coronaviruses, including SARS-CoV and SARS-CoV-2, were shown to undergo a quasispecies evolutionary process.
https://www.sciencedirect.com/science/article/pii/S0042682207001766
https://link.springer.com/article/10.1186/1471-2148-9-52
https://www.pnas.org/content/102/7/2430.short
This process postulates that there is no specific preadaptation of the virus to the host but instead a post-exposure, host-driven selection of viruses displaying the best propensity to evade immune surveillance and replicate. Contact, low affinity receptor interaction and lack of molecular interference during replication are enough to establish productive infection after what the virus will follow in-host selection.
This is compatible with the high diversity observed in the spike proteins of coronaviruses which is under positive selection ie host driven.
https://www.nature.com/articles/s41591-020-0820-9?fbclid=IwAR1Nj6E-XsU_N6IrFN1m9gCT-Q7app0iO2eUpN5x7OSi-l_q6c1LBx8-N24
https://link.springer.com/article/10.1186/1471-2148-9-52
https://mbio.asm.org/content/5/3/e01146-14?socid=social_ionutilize_20140709_24875696
https://www.nejm.org/doi/full/10.1056/NEJMc032421
In line with this model, the RBD from SARS-CoV-2 is not fully optimized for human ACE2 According to the circulation model, what really prepares the ground for the epidemic is simply an accidental event, i.e. a mutation, recombination or reassortment in the virus genome.
https://www.sciencedirect.com/science/article/pii/S1567134820303245#bb0015
The researchers say that “According to the circulation model, what really prepares the ground for the epidemic is simply an accidental event, i.e., a mutation, recombination or reassortment in the virus genome.”
The study team feels that the actual triggers for epidemics and pandemics lie in the organization of the society and human/animal contacts, and amplification loops offered by the human society, such as land conversion, contacts, markets, mobility, and international trade.
A significant positive aspect of the circulation model is that the focus of the model is on human activities and not wildlife, and if we want to prevent future pandemics like these, we must reconsider the manner in which we interact with nature.
The study team concluded that bats, pangolins, and other animals are not responsible for the epidemics or pandemics humans are facing right now. Blaming such infectious diseases on zoonotic emergence caused by wildlife may only result in unnecessary culling and mass slaughter leading to loss of biodiversity.
For the latest
SARS-CoV-2 News, keep on logging to Thailand Medical News.

