SARS-CoV-2 Nucleocapsid Protein Affects Cellular Defense by Targeting the Nonsense-Mediated mRNA Decay (NMD) Pathway
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 23, 2025 3 months, 2 weeks, 3 days, 1 hour, 48 minutes ago
Medical News: The world continues to study the intricate ways in which the SARS-CoV-2 virus, responsible for the COVID-19 pandemic, interacts with human cellular systems. A recent investigation sheds light on how the virus's nucleocapsid protein (Np) interferes with cellular defense mechanisms, particularly targeting the nonsense-mediated mRNA decay (NMD) pathway. The study, a collaborative effort among researchers from institutions including the Université Claude Bernard Lyon 1 in France, Sapienza University of Rome, and the University of Oxford, provides groundbreaking insights into the molecular strategies employed by SARS-CoV-2 to enhance its survival and replication.
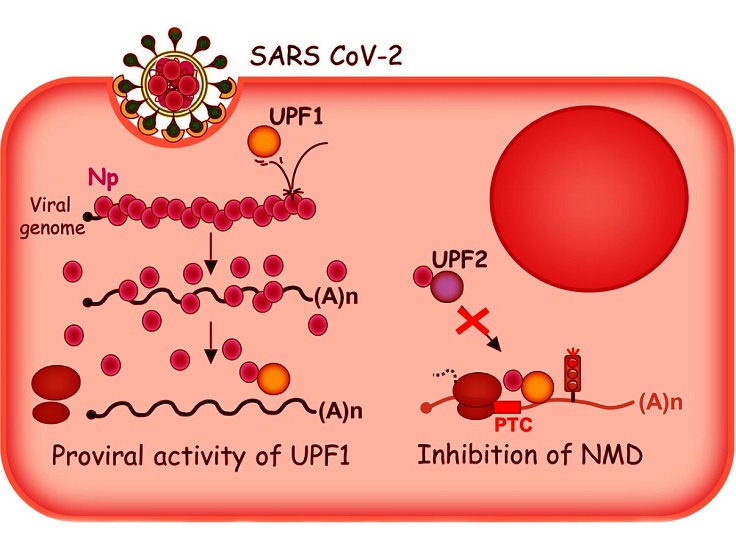 Graphical Abstract - SARS-CoV-2 Nucleocapsid Protein Affects Cellular Defense by Targeting the Nonsense-Mediated mRNA Decay (NMD) Pathway
Graphical Abstract - SARS-CoV-2 Nucleocapsid Protein Affects Cellular Defense by Targeting the Nonsense-Mediated mRNA Decay (NMD) Pathway
NMD serves as a quality control system in human cells, ensuring that defective mRNA molecules are identified and destroyed before they can be translated into dysfunctional proteins. During viral infections, however, this process is often disrupted. This
Medical News report highlights how SARS-CoV-2 exploits this mechanism to protect its RNA and promote its replication.
What Are UPF1 and UPF2?
UPF1 and UPF2 are critical components of the NMD pathway. UPF1, or "Up-frameshift 1," is a multifunctional RNA helicase enzyme essential for identifying and degrading faulty mRNA molecules. This enzyme plays a pivotal role by binding to RNA substrates and using energy derived from ATP hydrolysis to unwind RNA structures, allowing for proper inspection and processing. UPF1 is also involved in several other RNA-related processes, making it a central player in maintaining cellular RNA integrity.
UPF2, on the other hand, is a regulatory protein that interacts with UPF1 to enhance its enzymatic activity. The binding of UPF2 to UPF1’s CH domain activates UPF1, transitioning it from an RNA-clamping mode to an active translocation mode. This interaction is crucial for NMD to progress effectively. Together, UPF1 and UPF2 form a complex with other proteins to identify mRNA molecules flagged for degradation, ensuring that only healthy, functional transcripts are translated into proteins.
Key Findings from the Study
Researchers conducted biochemical and cellular assays to explore the interaction between the SARS-CoV-2 nucleocapsid protein and UPF1, a critical enzyme in the NMD pathway. The findings were startling:
-Direct Interaction with UPF1: The nucleocapsid protein binds directly to UPF1, inhibiting its enzymatic activation and ability to unwind RNA molecules. This prevents the normal progression of NMD.
-Impact on UPF2 Binding: The study further revealed that the nucleocapsid protein disrupts the interaction between UPF1 and UPF2, another essential component of the NMD pathway. By binding to UPF2, the viral protein reduces the formation of UPF1-UPF2 complexes necessary for NMD.
-Stabilization of NMD-Prone RNA: Experimental data showed that the presence of the nucleocapsid protein significantly stabilizes mRNA molecules that would typically be degraded by NMD. This stabilization likely allows the virus to manipulate host cellular machinery for its benefit.
-Dual Roles of UPF1 and UPF2: While UPF1 appears to have a pro-viral role by promoting viral RNA stability and translation, UPF2 exhibits an antiviral function by facilitating the decay of viral RNA. The nucleocapsid protein’s interference with these interactions creates an environment favorable for viral replication.
Detailed Mechanisms of Inhibition
The study employed advanced techniques, including microscale thermophoresis and co-immunoprecipitation, to investigate the molecular interactions in detail. Researchers observed that the nucleocapsid protein binds preferentially to structured regions of RNA substrates, effectively blocking UPF1 from accessing these areas. Interestingly, this interaction does not affect UPF1's ATPase activity or its ability to translocate along RNA molecules. Instead, it specifically inhibits the unwinding of RNA duplexes - a critical step in NMD.
In cell-based experiments, overexpression of the nucleocapsid protein was shown to reduce the degradation of mRNA reporters targeted by NMD. This was corroborated by observing increased levels of these mRNA molecules in infected cells. Notably, the inhibition of NMD was reversible; overexpression of UPF2 partially restored normal mRNA decay, demonstrating its antiviral role.
The Broader Role of NMD in Viral Infections
NMD is not only a quality control system but also a defense mechanism against RNA viruses like SARS-CoV-2. Many viruses have evolved strategies to counteract or evade NMD. For instance, the nucleocapsid proteins of several coronaviruses, including SARS-CoV-2, directly interact with NMD components to suppress this pathway. By stabilizing viral RNA and preventing its degradation, the virus ensures that its genetic material remains available for replication and protein production.
Interestingly, some viruses exploit NMD factors for their benefit. For example, HIV-1 hijacks UPF1 to stabilize its RNA and enhance the production of viral proteins. This dual role of NMD components - acting as both antiviral and pro-viral factors depending on the context - underscores the complexity of virus-host interactions.
Implications for SARS-CoV-2 Replication
The study also explored the impact of NMD inhibition on viral replication. Using a replicative strain of SARS-CoV-2 containing a fluorescent reporter, researchers found that cells infected with the virus exhibited suppressed NMD activity.
Additionally, they discovered that overexpression of UPF1 slightly enhanced viral replication, while overexpression of UPF2 reduced it. These findings highlight the complex interplay between viral strategies and host defenses.
The dual roles of UPF1 and UPF2 suggest that while the virus co-opts UPF1 for its benefit, it must simultaneously neutralize the antiviral effects of UPF2. This delicate balance underscores the evolutionary sophistication of SARS-CoV-2 and provides potential avenues for therapeutic intervention.
Potential Therapeutic Strategies
Understanding the mechanisms by which SARS-CoV-2 manipulates NMD opens up new possibilities for antiviral therapies. Targeting the interaction between the nucleocapsid protein and NMD components could restore normal cellular functions and inhibit viral replication. For example, small molecules or peptides designed to block the binding sites of the nucleocapsid protein on UPF1 and UPF2 could prevent the virus from hijacking these factors.
Additionally, enhancing the expression or activity of UPF2 could bolster the cell’s antiviral defenses. Given UPF2’s role in promoting the decay of viral RNA, strategies to increase its availability in infected cells may limit the replication and spread of the virus.
Conclusions and Future Directions
The findings of this study provide a detailed understanding of how SARS-CoV-2 manipulates the NMD pathway to evade cellular defenses and enhance its replication. By targeting the interactions between the nucleocapsid protein and key components of the NMD pathway, new therapeutic strategies can be developed to disrupt this mechanism and potentially mitigate the effects of COVID-19.
Future research should focus on identifying small molecules or inhibitors that can prevent the nucleocapsid protein from binding to UPF1 and UPF2. Additionally, understanding the broader implications of NMD inhibition in SARS-CoV-2 infections could reveal insights into the virus's impact on host cellular processes beyond RNA stability.
The study findings were published in the peer-reviewed journal: Nucleic Acids Research.
https://academic.oup.com/nar/article/53/2/gkaf010/7962007
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-nucleocapsid-protein-prevents-viral-degradation-by-disrupting-nonsense-mediated-mrna-decay
https://www.thailandmedical.news/news/new-insights-into-sars-cov-2-nsp12-activity
https://www.thailandmedical.news/news/sars-cov-2-disrupts-the-hemostatic-system-and-the-complement-system-in-a-complex-interplay
https://www.thailandmedical.news/articles/coronavirus
