Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 15, 2024 1 year, 1 month, 1 week, 3 days, 18 hours, 54 minutes ago
Medical News: Scientists Uncover New Insights Into COVID-19 and Cholesterol
A groundbreaking study has revealed how SARS-CoV-2, the virus behind COVID-19, disrupts cholesterol transport within human cells. Researchers from the University of New Mexico Health Sciences Center-USA and UT Health San Antonio Long School of Medicine-USA discovered that the virus's ORF3a protein interferes with cellular systems managing cholesterol distribution, potentially contributing to the metabolic complications associated with COVID-19.
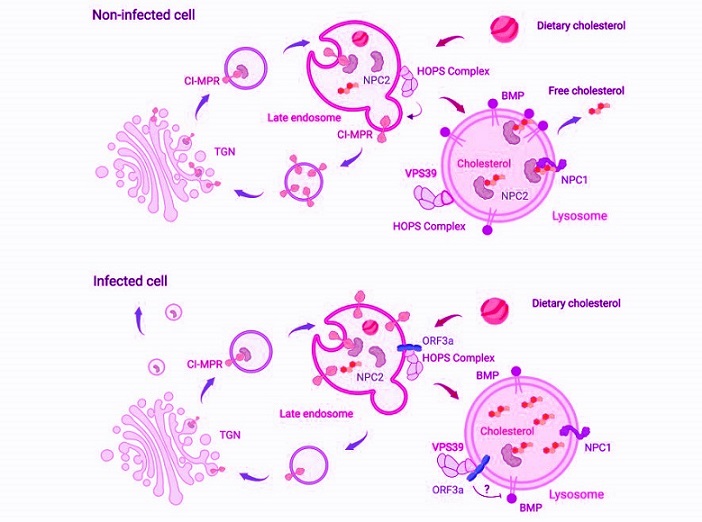 SARS-CoV-2’s ORF3a protein manipulates cholesterol levels in the host
Scheme of the pathways participating in lysosomal cholesterol egress. In healthy cells, lysosomes process endocytosed low-density lipoproteins (LDLs) to release free cholesterol, which is exported via the cholesterol transporters Niemann-Pick C 2 (NPC2) and NPC1. NPC2 also binds with the phospholipid bis(monoacylglycero)phosphate (BMP) to export cholesterol independently of NPC1. Newly synthesized NPC2 associates with the sorting receptor mannose-6-phosphate receptors, such as CI-MPR, in the trans-Golgi network (TGN) and is delivered to late endosomes. Here, CI-MPR is retrieved and recycled back to the TGN with help from the HOPS complex. In SARS-CoV-2-infected cells, ORF3a interacts with the HOPS subunit VPS39, disrupting CI-MPR recycling. This leads to NPC2 trafficking defects, i.e., mislocalization and increased secretion. Additionally, the ORF3a-VPS39 interaction reduces BMP levels by an undefined mechanism, together resulting in cholesterol sequestration within lysosomes
SARS-CoV-2’s ORF3a protein manipulates cholesterol levels in the host
Scheme of the pathways participating in lysosomal cholesterol egress. In healthy cells, lysosomes process endocytosed low-density lipoproteins (LDLs) to release free cholesterol, which is exported via the cholesterol transporters Niemann-Pick C 2 (NPC2) and NPC1. NPC2 also binds with the phospholipid bis(monoacylglycero)phosphate (BMP) to export cholesterol independently of NPC1. Newly synthesized NPC2 associates with the sorting receptor mannose-6-phosphate receptors, such as CI-MPR, in the trans-Golgi network (TGN) and is delivered to late endosomes. Here, CI-MPR is retrieved and recycled back to the TGN with help from the HOPS complex. In SARS-CoV-2-infected cells, ORF3a interacts with the HOPS subunit VPS39, disrupting CI-MPR recycling. This leads to NPC2 trafficking defects, i.e., mislocalization and increased secretion. Additionally, the ORF3a-VPS39 interaction reduces BMP levels by an undefined mechanism, together resulting in cholesterol sequestration within lysosomes
This
Medical News report sheds light on the intricate mechanisms SARS-CoV-2 employs to exploit host cholesterol pathways. Cholesterol plays an essential role in maintaining cell membrane structure and regulating various cellular processes. By manipulating these pathways, the virus not only enhances its infectivity but also alters the host's metabolic balance.
What Did the Study Find?
The researchers found that SARS-CoV-2 infection leads to cholesterol accumulation in lysosomes - specialized cellular compartments responsible for breaking down waste and recycling nutrients. Normally, cholesterol is transported out of lysosomes and distributed to other parts of the cell. However, the viral ORF3a protein disrupts this process by interacting with a cellular protein called VPS39, a key component of the HOPS complex, which governs lysosomal trafficking.
This interaction causes a cascade of effects, including reduced levels of a lipid called bis(monoacylglycero)phosphate (BMP), which is critical for cholesterol transport. As a result, cholesterol gets trapped in lysosomes, disrupting normal cellular functions.
The Role of the ORF3a Protein
To pinpoint the specific viral components responsible, the research team screened 28 SARS-CoV-2 proteins. ORF3a stood out as the primary culprit. This protein not only caused lysosomal cholesterol acc
umulation but also disrupted the trafficking of NPC2, a protein crucial for cholesterol export from lysosomes. Additionally, ORF3a altered the distribution of cellular sorting receptors like CI-MPR and TGN46, further compounding the cholesterol transport defects.
Interestingly, the study compared ORF3a from SARS-CoV-2 with its counterpart in the original SARS virus. While both proteins shared structural similarities, only the SARS-CoV-2 version caused significant cholesterol sequestration. This difference may offer clues about why COVID-19 has unique metabolic impacts compared to SARS.
How Cholesterol Alteration Affects Viral Behavior
The study suggests that trapping cholesterol in lysosomes might serve a dual purpose for SARS-CoV-2. On the one hand, it reduces cholesterol at the cell surface, potentially limiting secondary infections of the same cell. On the other hand, it creates a more favorable environment for viral replication. Experiments using a compound that mimicked this cholesterol sequestration showed a significant decrease in viral infection, hinting at potential therapeutic strategies.
Broader Implications for COVID-19 Patients
Cholesterol dysregulation has been observed in COVID-19 patients, with studies linking low serum cholesterol levels to disease severity. The findings from this study provide a cellular explanation for these observations. By hijacking cholesterol transport systems, SARS-CoV-2 could contribute to the lipid imbalances seen in long COVID, including increased risks of cardiovascular diseases.
Future Directions and Potential Treatments
Understanding the interplay between SARS-CoV-2 and host lipid metabolism opens new avenues for treatment. Targeting the ORF3a-VPS39 interaction or restoring BMP levels in lysosomes could help mitigate the virus's effects on cholesterol metabolism. These strategies may not only reduce the severity of COVID-19 but also address its long-term metabolic consequences.
Conclusion
This study underscores the sophisticated ways in which SARS-CoV-2 manipulates host cellular processes to its advantage. By disrupting cholesterol transport, the virus not only ensures its survival but also triggers widespread metabolic disturbances. Addressing these disruptions could be key to improving outcomes for COVID-19 patients.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.11.13.623299v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-causes-blood-fat-disorders
https://www.thailandmedical.news/news/russian-study-uncovers-cholesterol-s-critical-role-in-sars-cov-2-e-protein-function
