SARS-CoV-2 Spike Glycoprotein Interacts With Monoamine Oxidase B And Impairs Mitochondrial Energetics Causing Neurodegenerative Issues!
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 08, 2023 2 years, 2 months, 2 weeks, 16 hours, 26 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has not only affected the respiratory system but has also revealed a significant impact on the neurological health of individuals. While the primary focus has been on acute respiratory symptoms, emerging evidence suggests a link between SARS-CoV-2 infection and various neurological complications. One intriguing aspect of this link is the potential involvement of mitochondrial dysfunction and the monoamine oxidase B (MAO-B) enzyme in the development of neurodegenerative issues following COVID-19 recovery.
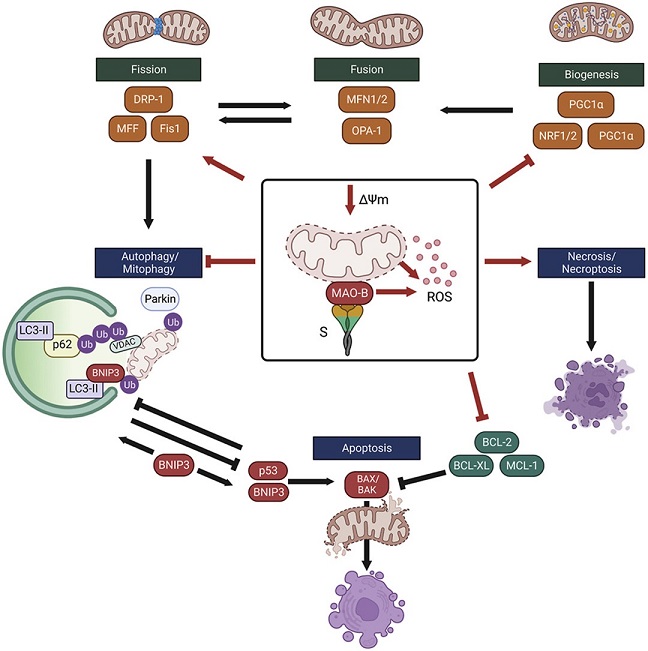 Schematic summary of the SARS-CoV-2 spike glycoprotein-induced impairments in mitochondrial health that contribute to increased susceptibility to MPTP-induced cell death. Red lines indicate changes induced by expression of the SARS-CoV-2 spike glycoprotein in SH-SY5Y neuron-like cells. The S protein depletes mitochondria membrane potential and enhances MAO-B activity and ROS production. Expression of mitochondrial transcription factors are lower in neuron-like cells expressing the S protein. Degradation of depolarized mitochondria by mitophagy is inhibited, in part by lower expression of the E3 ligase, parkin, leading to the accumulation of depolarized aberrant mitochondria. Low expression of BCL-2 anti-apoptotic proteins and activation of necrosis/necroptosis increases the susceptibility to cell death. Comparisons between groups with a two-way ANOVA with Holm-Sidak post-hoc test. Values are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. s.
Schematic summary of the SARS-CoV-2 spike glycoprotein-induced impairments in mitochondrial health that contribute to increased susceptibility to MPTP-induced cell death. Red lines indicate changes induced by expression of the SARS-CoV-2 spike glycoprotein in SH-SY5Y neuron-like cells. The S protein depletes mitochondria membrane potential and enhances MAO-B activity and ROS production. Expression of mitochondrial transcription factors are lower in neuron-like cells expressing the S protein. Degradation of depolarized mitochondria by mitophagy is inhibited, in part by lower expression of the E3 ligase, parkin, leading to the accumulation of depolarized aberrant mitochondria. Low expression of BCL-2 anti-apoptotic proteins and activation of necrosis/necroptosis increases the susceptibility to cell death. Comparisons between groups with a two-way ANOVA with Holm-Sidak post-hoc test. Values are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. s.
This
COVID-19 News report delves into a study conducted by researchers from the University of Ottawa in Canada and the National Research Council of Canada, in collaboration with the Centre for Infection, Immunity, and Inflammation, Ottawa. Their research explores the interaction between the SARS-CoV-2 spike (S) glycoprotein and MAO-B, shedding light on how this interaction could impair mitochondrial energetics and contribute to neurodegenerative problems.
Neurological Implications of SARS-CoV-2 Infection
SARS-CoV-2 primarily infects the respiratory tract, leading to pneumonia-like symptoms. However, it has become increasingly evident that neurological symptoms are prevalent in COVID-19 patients, with up to 70-80% of hospitalized patients experiencing them. Although most neurological symptoms resolve following the initial infection, a subset of patients reports persistent neurocognitive issues, including a condition known as "long-COVID."
Evidence of SARS-CoV-2 neurotropism has been observed in brain tissue from deceased COVID-19 patients, where viral antigens and RNA were detected in neurons, microglia, and astrocytes. This cerebral infection is associated with a wide range of acute and chronic pathological changes, including cerebrovascular damage, infarcts, hypometabolism, brain size reduction, and decreased gray matter thickness. These findings emphasize the complex molecular mechanisms behind acute and persistent neurological symptoms in COVID-19 patients.
SARS-CoV-2 Spike Glycoprotein and N
euroinvasion
The SARS-CoV-2 spike (S) protein plays a pivotal role in viral entry by facilitating the fusion between the virus and host cell membranes. To enter host cells, the S protein must undergo proteolytic processing and bind to the angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 receptors are expressed in cells that line the blood-brain barrier (BBB) and the choroid plexus epithelium, making them potential entry points for the virus into the central nervous system (CNS).
Olfactory neurons, which have lower ACE2 expression, may also contribute to CNS entry through neuropilin-1 or transmission from non-neuronal olfactory cells with high ACE2 expression. The S1 subunit of the S protein can cross the BBB, potentially contributing to the development of neurological symptoms. These findings highlight the potential for SARS-CoV-2 to directly affect brain metabolism and function.
Mitochondrial Dysfunction and SARS-CoV-2
Increasing evidence suggests that SARS-CoV-2 targets mitochondria, leading to disruptions in cellular energy metabolism that resemble those seen in neurodegenerative diseases. SARS-CoV-2 infection is associated with changes in mitochondrial morphology, alterations in bioenergetic function, increased production of reactive oxygen species (ROS), and decreased mitochondrial membrane potential. Intriguingly, the S protein can interact with cellular proteins and modulate various cellular processes independently of other viral components, including mitochondrial function.
Monoamine Oxidase B (MAO-B) and Its Role in Neurodegeneration
Researchers have discovered a remarkable structural similarity between the SARS-CoV-2 spike glycoprotein receptor binding domain on the ACE2 receptor and MAO-B, an enzyme located on the outer mitochondrial membrane. MAO-B is responsible for the oxidative deamination of monoamine neurotransmitters, such as dopamine and 2-phenylethylamine. Oxidation of MAO-B substrates generates hydrogen peroxide (H2O2), which can lead to cell death and mitochondrial dysfunction.
Elevated MAO-B activity is associated with neurodegenerative diseases, particularly Parkinson's disease, where it contributes to increased ROS production, mitochondrial dysfunction, and dopaminergic neuron loss. As a result, MAO-B inhibitors are used therapeutically to mitigate neurodegeneration in Parkinson's disease.
Computational modeling suggests that the interaction between the S protein and MAO-B may alter neurotransmitter metabolism by affecting neurotransmitter access to the MAO-B active binding site or modifying the electrostatic environment. COVID-19 patients show increased platelet MAO-B gene expression, indicating dysregulated monoamine metabolism. MAO-B was also found to be one of the most upregulated genes in the blood of patients with varying degrees of COVID-19 severity.
Emerging evidence even suggests a potential link between SARS-CoV-2 and post-encephalitic Parkinsonism, reminiscent of the consequences of the Spanish influenza in 1918. Thus, understanding the neurological ramifications of SARS-CoV-2 infection is of paramount importance.
Interaction between SARS-CoV-2 Spike Glycoprotein and MAO-B
The primary objective of this study was to investigate whether the SARS-CoV-2 S protein interacts with MAO-B and alters MAO-B activity, potentially sensitizing neuron-like cells to neurodegeneration resembling Parkinson's disease.
The results of the study revealed that indeed, the SARS-CoV-2 S protein interacts with cellular MAO-B and augments its activity. Furthermore, the S protein disrupts mitophagy, leading to the accumulation of dysfunctional mitochondria. Neuron-like cells expressing the S protein also exhibited increased susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced necrosis, a phenomenon associated with necroptosis.
Discussion
The study's findings highlight the potential mechanisms underlying SARS-CoV-2-induced neurodegeneration. Several key points can be summarized:
-SARS-CoV-2 S protein interacts with and enhances MAO-B activity.
-The S protein disrupts mitochondrial bioenergetics, induces oxidative stress, and impairs mitophagy.
-Neuron-like cells expressing the S protein are more susceptible to MPTP-induced necrosis.
-These observations are consistent with the pathophysiological mechanisms seen in neurodegenerative diseases.
Parkinson's disease, characterized by the progressive loss of dopaminergic neurons and the accumulation of misfolded alpha-synuclein leading to Lewy body pathology, is known to exhibit elevated MAO-B activity. High MAO-B activity increases dopamine catabolism and the formation of DOPAL, contributing to the aggregation of alpha-synuclein. MAO-B inhibitors are an effective treatment for alleviating Parkinson's motor symptoms.
While substantial brain invasion by SARS-CoV-2 is relatively rare due to low ACE2 expression, some brain regions, including the substantia nigra, have higher ACE2 expression. This brain invasion can result in the onset of Parkinsonism following SARS-CoV-2 infection, along with disturbances in nigrostriatal dopamine function. COVID-19 patients with pre-existing Parkinson's disease often require increased levodopa dosing, indicating elevated dopamine metabolism following SARS-CoV-2 infection. Neurodegeneration and Parkinson's disease-related pathways were also enriched in proteomic analysis of post-mortem brain tissue from deceased COVID-19 patients.
The S protein's interaction with MAO-B and its potential effects on neurotransmitter metabolism may contribute to these observed neurodegenerative changes. Computational modeling suggests that the electrostatic environment of the MAO-B substrate binding site could be modified by the S protein. Additionally, different SARS-CoV-2 strains may have varying impacts on MAO-B activity, possibly influenced by their proximity to lipid membranes.
Altered monoamine metabolism in SARS-CoV-2 infection may be amplified by the downregulation of L-DOPA decarboxylase (DDC), further implicating MAO-B in the development of neurological symptoms. High levels of psychological distress before infection are also associated with long-COVID neuropsychiatric symptoms, such as fatigue and cognitive impairment.
The production of H2O2 through MAO-B activity is directly linked to mitochondrial dysfunction, as it impairs mitochondrial function and promotes dopaminergic neuron cell death. In line with this, the study found elevated rates of mitochondrial H2O2 emissions and decreased glutathione content in cells expressing the S protein. These changes may contribute to oxidative stress, a major contributor to neurodegeneration in Parkinson's disease.
Moreover, the study revealed that neuron-like cells expressing the S protein exhibited increased susceptibility to MPTP-induced neurotoxicity. MAO-B's role in catalyzing the conversion of MPTP to MPP+ in astrocytes, followed by its uptake into dopaminergic neurons, suggests that enhanced MAO-B activity could contribute to neurotoxicity.
Conclusion
In conclusion, this study provides valuable insights into the potential mechanisms by which SARS-CoV-2 may induce neurodegeneration and alter monoamine metabolism. The interaction between the S protein and MAO-B, as well as its impact on mitochondrial function and sensitivity to neurotoxic insults, highlights the intricate relationship between viral infection and neurological complications. Further research is warranted to explore the therapeutic potential of MAO-B inhibitors in preventing or mitigating SARS-CoV-2-induced neurodegeneration, ultimately improving the quality of life for COVID-19 survivors.
The study findings were published in the peer reviewed journal: Current Research in Neurobiology.
https://www.sciencedirect.com/science/article/pii/S2665945X23000402
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
