SARS-CoV-2 Spike Protein Impairs Gastrointestinal Tract And Affects Mucosal Barrier!
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 29, 2024 1 year, 2 weeks, 3 days, 12 hours, 8 minutes ago
COVID-19 News: In recent times, the world has witnessed the devastating effects of the COVID-19 pandemic caused by the SARS-CoV-2 virus. While the respiratory symptoms of the disease have garnered significant attention, emerging evidence suggests that the virus can also affect the gastrointestinal (GI) system, leading to symptoms such as diarrhea, abdominal pain, and nausea. Understanding the mechanisms underlying these GI manifestations is crucial for developing effective treatment strategies. In this
COVID-19 News report, we delve into the intricate interactions between the SARS-CoV-2 Spike protein and the GI tract, shedding light on how this viral component impairs the mucosal barrier and affects intestinal function.
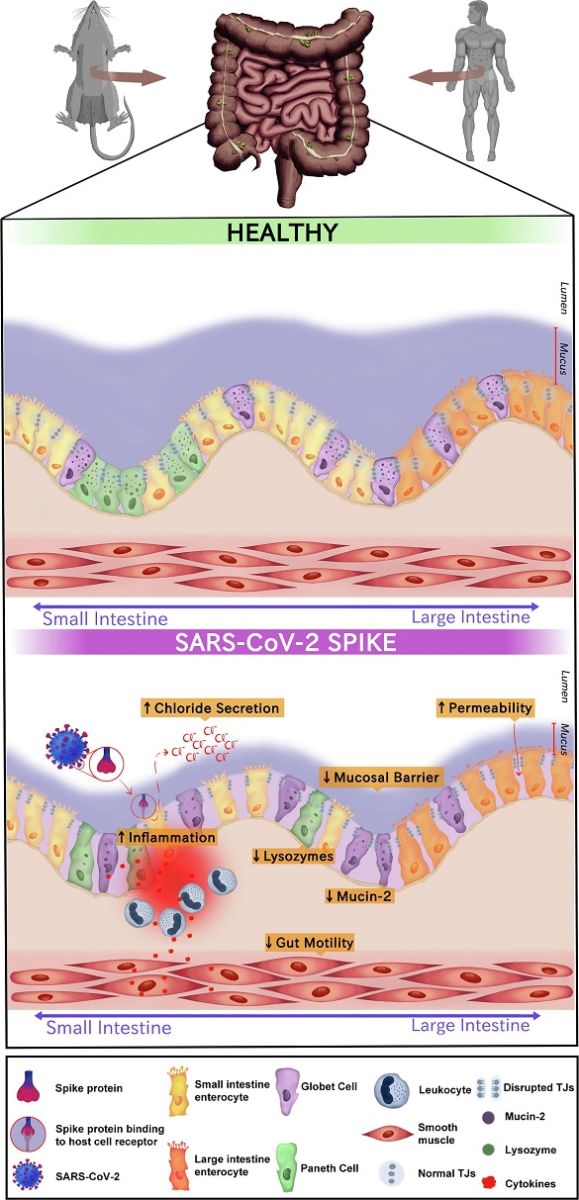 The Effects of SARS-CoV-2 Spike Protein on Gastrointestinal Health. The schematic figure illustrates the complex interactions between the SARS-CoV-2 Spike protein and the gastrointestinal (GI) tract, highlighting the observed effects in both rodent and human samples. 1) Augmented Chloride Secretion in Jejunal Segments: In rodent jejunal segments, exposure to the SARS-CoV-2 Spike protein triggers a remarkable increase in chloride (Cl-) secretion. This electrolyte imbalance disrupts the homeostatic environment of the small intestine (left side), potentially leading to altered fluid balance and diarrhea. 2) Impairment of Mucosal Integrity and Tight Junction Disruption: A novel murine model of enteritis induced by the SARS-CoV-2 Spike protein demonstrates a profound impact on mucosal integrity. The protein's presence leads to the disruption of tight junctions (TJs) between enterocytes, resulting in reduced transepithelial electrical resistance. This breach in barrier function may facilitate the translocation of harmful substances across the intestinal lining. 3) Increased Mucosal Permeability in Human Enterocytes (right side): In human enterocytes, exposure to the SARS-CoV-2 Spike protein leads to an increase in mucosal permeability. This compromised barrier function allows the passage of molecules that would normally be restricted, potentially contributing to immune responses. 4) Reduced Mucus Blanket and MUC-2 Depletion: Examination of the jejunum reveals a marked reduction in the mucus blanket that typically coats the intestinal surface. Goblet cells, responsible for mucus production, show decreased bulk in MUC-2, a key component of mucus. This reduction may compromise the protective barrier provided by mucus, leaving the intestinal epithelium vulnerable to pathogenic insults. 5) Paneth Cell Degranulation and Lysozyme Depletion: SARS-CoV-2 inoculation in the luminal gut environment triggers Paneth cell degranulation. These specialized cells release antimicrobial molecules such as lysozymes, which play a crucial role in maintaining gut microbial balance. However, the presence of the Spike protein results in a notable reduction in lysozyme levels, potentially affecting the gut's ability to control microbial populations. 6) Increased Expression of Cytokines, Especially IL-6: The exposure to the SARS-CoV-2 Spike protein induces heightened expression of cytokines, notably in
terleukin-6 (IL-6). This pro-inflammatory cytokine may contribute to gut tissue damage, including innermost layers such as smooth muscle, and influencing the overall immune response. 7) Reduced Gut Motility: The cumulative effects of the SARS-CoV-2 Spike protein on the GI tract culminate in reduced gut motility. This disruption may stem from the combined impact of disrupted electrolyte balance, compromised mucosal integrity, and heightened cytokine, mainly IL-6, expression. Altered gut motility can have cascading effects on digestion, absorption, and overall GI function. The legend underscores the intricate cascade of events triggered by the SARS-CoV-2 Spike protein's interaction with the gastrointestinal system, highlighting the potential for significant disruptions in gut health and homeostasis. These findings shed light on the broader impact of the virus beyond the respiratory system, emphasizing the need for comprehensive understanding and targeted interventions.
Introduction: Unraveling the GI Impact of COVID-19
The Effects of SARS-CoV-2 Spike Protein on Gastrointestinal Health. The schematic figure illustrates the complex interactions between the SARS-CoV-2 Spike protein and the gastrointestinal (GI) tract, highlighting the observed effects in both rodent and human samples. 1) Augmented Chloride Secretion in Jejunal Segments: In rodent jejunal segments, exposure to the SARS-CoV-2 Spike protein triggers a remarkable increase in chloride (Cl-) secretion. This electrolyte imbalance disrupts the homeostatic environment of the small intestine (left side), potentially leading to altered fluid balance and diarrhea. 2) Impairment of Mucosal Integrity and Tight Junction Disruption: A novel murine model of enteritis induced by the SARS-CoV-2 Spike protein demonstrates a profound impact on mucosal integrity. The protein's presence leads to the disruption of tight junctions (TJs) between enterocytes, resulting in reduced transepithelial electrical resistance. This breach in barrier function may facilitate the translocation of harmful substances across the intestinal lining. 3) Increased Mucosal Permeability in Human Enterocytes (right side): In human enterocytes, exposure to the SARS-CoV-2 Spike protein leads to an increase in mucosal permeability. This compromised barrier function allows the passage of molecules that would normally be restricted, potentially contributing to immune responses. 4) Reduced Mucus Blanket and MUC-2 Depletion: Examination of the jejunum reveals a marked reduction in the mucus blanket that typically coats the intestinal surface. Goblet cells, responsible for mucus production, show decreased bulk in MUC-2, a key component of mucus. This reduction may compromise the protective barrier provided by mucus, leaving the intestinal epithelium vulnerable to pathogenic insults. 5) Paneth Cell Degranulation and Lysozyme Depletion: SARS-CoV-2 inoculation in the luminal gut environment triggers Paneth cell degranulation. These specialized cells release antimicrobial molecules such as lysozymes, which play a crucial role in maintaining gut microbial balance. However, the presence of the Spike protein results in a notable reduction in lysozyme levels, potentially affecting the gut's ability to control microbial populations. 6) Increased Expression of Cytokines, Especially IL-6: The exposure to the SARS-CoV-2 Spike protein induces heightened expression of cytokines, notably in
terleukin-6 (IL-6). This pro-inflammatory cytokine may contribute to gut tissue damage, including innermost layers such as smooth muscle, and influencing the overall immune response. 7) Reduced Gut Motility: The cumulative effects of the SARS-CoV-2 Spike protein on the GI tract culminate in reduced gut motility. This disruption may stem from the combined impact of disrupted electrolyte balance, compromised mucosal integrity, and heightened cytokine, mainly IL-6, expression. Altered gut motility can have cascading effects on digestion, absorption, and overall GI function. The legend underscores the intricate cascade of events triggered by the SARS-CoV-2 Spike protein's interaction with the gastrointestinal system, highlighting the potential for significant disruptions in gut health and homeostasis. These findings shed light on the broader impact of the virus beyond the respiratory system, emphasizing the need for comprehensive understanding and targeted interventions.
Introduction: Unraveling the GI Impact of COVID-19
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has posed unprecedented challenges to global health systems. Initially recognized for its respiratory symptoms, including cough, fever, and pneumonia, COVID-19 has also been associated with a range of GI symptoms. These symptoms, such as diarrhea, vomiting, and abdominal discomfort, have been reported in a significant proportion of COVID-19 patients and can occur independently of respiratory symptoms or precede them.
The impact of SARS-CoV-2 on the GI tract has garnered increasing attention due to its implications for disease prognosis and management. Patients presenting with GI symptoms often experience a prolonged illness course and may have a higher risk of complications. Moreover, the presence of the virus in fecal samples and its potential for fecal-oral transmission highlight the importance of understanding how SARS-CoV-2 interacts with the GI system.
Investigating SARS-CoV-2 Spike Protein in the GI Context
A collaborative effort involving researchers from various institutions sought to investigate the specific role of the SARS-CoV-2 Spike protein (Spk) in inducing GI pathology. Researchers from the Federal University of Ceará-Brazil, University of Virginia School of Medicine-USA, Faculdades Pequeno Príncipe-Brazil, Parnaíba Delta Federal University-Brazil, Federal University of Rondonópolis-Brazil, and Medical University of South Carolina-USA collaborated on this groundbreaking study.
Experimental Approaches and Findings
The research team employed a multi-pronged approach to assess the impact of the Spike protein on the GI tract. This included in vivo experiments using murine models, in vitro studies with human enterocytes, and molecular docking analysis to elucidate protein interactions.
The key findings of the study were:
-SARS-CoV-2 Spike protein (Spk) induces intestinal chloride secretion in mice;
-SARS-CoV-2 Spike protein causes lysozymes depletion by Paneth cells degranulation;
-SARS-CoV-2 Spike protein diminishes mucus blanket associated with mucin 2 reduction;
-The mucosal intestinal barrier is impaired by SARS-CoV-2 Spike protein inoculation on the gut;
-SARS-CoV-2 Spike protein triggers local inflammation and dysmotility on the gut.
The Spike protein of SARS-CoV-2 triggered a cascade of events within the gastrointestinal (GI) tract, starting with an increase in intestinal fluid and chloride (Cl-) secretion. This led to intestinal edema, infiltration of leukocytes, and a decrease in glutathione levels, along with elevated levels of inflammatory cytokines such as IL-6, TNF-α, IL-1β, and IL-10, indicating the onset of inflammation. Moreover, the viral epitope disrupted the mucosal histoarchitecture, particularly affecting Paneth and goblet cells, resulting in reduced lysozyme and mucin production, respectively.
Furthermore, the upregulation of TLR2 and TLR4 gene expression hinted at the activation of local innate immunity in response to the Spike protein. This immune response was accompanied by reduced contractile responses in the smooth muscle of the jejunal region. Additionally, alterations in barrier function were observed, including decreased transepithelial electrical resistance and changes in the expression of tight junction proteins in the murine jejunal epithelium. These changes contributed to an increase in paracellular intestinal permeability, as evidenced by experiments using human enterocytes
Inflammatory Changes Triggered by SARS-CoV-2 Spike Protein
One of the key findings of the study was the inflammatory response elicited by the Spike protein in the GI mucosa. Upon exposure to the Spike protein, there was a notable increase in intestinal fluid secretion, primarily driven by chloride ions. This secretory effect was accompanied by edema, leukocyte infiltration, and altered cytokine levels, indicating a local inflammatory response within the intestinal tissue.
Impact on Intestinal Cell Types
Further investigation revealed specific alterations in intestinal cell types following Spike protein exposure. Paneth cells, vital for antimicrobial defense, exhibited degranulation, leading to a reduction in lysozyme levels. Similarly, goblet cells responsible for mucus production showed diminished expression of mucin 2 (MUC2), a crucial component of the mucosal barrier. These changes collectively contributed to mucosal disruption and compromised barrier function.
Functional Changes in Intestinal Tissues
Functional analyses demonstrated that the Spike protein not only affected cellular viability but also increased intestinal permeability. In vitro experiments with human enterocytes showed reduced cell viability and enhanced paracellular permeability upon Spike protein exposure. These findings underscored the direct impact of the Spike protein on intestinal integrity and barrier function.
Molecular Docking Insights
Molecular docking studies provided further insights into the mechanisms underlying the Spike protein's effects. The protein was found to interact with calcium and chloride channel proteins, suggesting a direct role in ion transport and secretory processes within intestinal epithelial cells. This molecular interaction likely contributes to the observed secretory diarrhea associated with COVID-19.
Discussion and Implications
The study's findings offer a comprehensive understanding of how the SARS-CoV-2 Spike protein impairs the GI tract at multiple levels. From inducing inflammation and altering cellular functions to disrupting mucosal integrity and promoting secretory diarrhea, the Spike protein emerges as a key player in GI pathology associated with COVID-19.
Translational Relevance and Future Directions
The establishment of a robust experimental model for studying GI manifestations of COVID-19 lays the foundation for future translational research. Targeting the mechanisms identified in this study could lead to the development of novel therapeutic interventions aimed at mitigating GI complications in COVID-19 patients.
Conclusion
In conclusion, the SARS-CoV-2 Spike protein exerts a significant impact on the gastrointestinal tract, disrupting mucosal integrity, inducing inflammation, and altering cellular functions. This comprehensive investigation sheds light on the pathobiology of COVID-19 in the GI context and paves the way for targeted interventions to alleviate GI symptoms and improve patient outcomes. Further research aimed at unraveling the intricacies of viral-host interactions in the GI tract holds immense promise for advancing our understanding and management of COVID-19-related gastrointestinal complications.
The study findings were published in the peer reviewed journal: Mucosal Immunology.
https://www.sciencedirect.com/science/article/pii/S1933021924000291
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
