SARS-CoV-2 Uses Phase Separation to Evade Immune Defenses: New Insights and Potential Treatments
Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 25, 2024 10 months, 1 day, 18 hours, 58 minutes ago
COVID-19 News:
Understanding How Coronaviruses Evade the Immune System
The Basics of Coronaviruses
Coronaviruses (CoVs) are a family of viruses that include the infamous SARS-CoV-2, responsible for the COVID-19 pandemic. These viruses can infect both humans and animals, leading to diseases ranging from the common cold to more severe respiratory illnesses. The structure of these viruses includes spike proteins, envelope proteins, membrane proteins, and nucleocapsid proteins, which play critical roles in the virus's life cycle.
What is Liquid-Liquid Phase Separation (LLPS)?
LLPS is a process where certain proteins and nucleic acids within cells separate into distinct liquid phases, forming droplets. This separation helps organize cellular components and regulate biological processes. Recent research by scientists from the School of Medicine, Jiangsu University, Zhenjiang-China that is covered in this
COVID-19 News report has uncovered that coronaviruses exploit LLPS to evade the host's immune system.
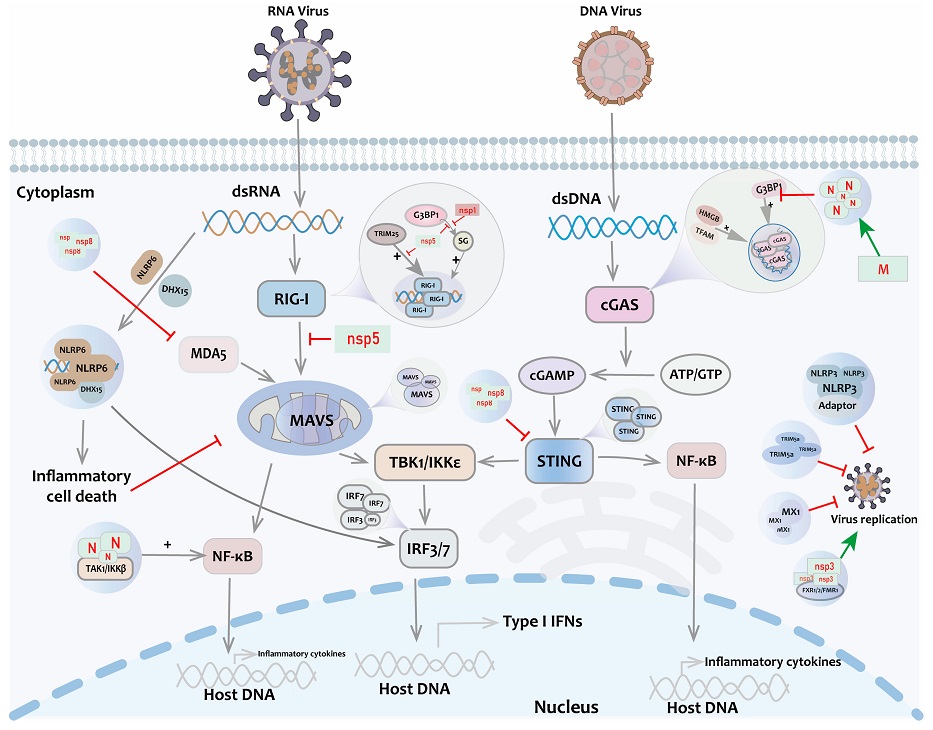 Liquid-Liquid Phase Separation (LLPS) in the innate immune pathway. After stimulation by viral RNA/DNA, key immune molecules such as RIG-I, MAVS, cGAS, IRF3, and STING undergo LLPS to activate IFN signaling. Positive regulation of antiviral response is also present with TRIM25, NEMO, TFAM, HMGB, etc. Antiviral factors such as TRIM5a, MX1, NLRP3, NLRP6, etc., form liquid-like condensates and mediate antiviral immune response. At the same time, non-structural proteins of coronaviruses such as Nsp5 and Nsp1 participate in the negative regulation of antiviral responses.
How Coronaviruses Use LLPS to Their Advantage
-LLPS in Viral Replication
Liquid-Liquid Phase Separation (LLPS) in the innate immune pathway. After stimulation by viral RNA/DNA, key immune molecules such as RIG-I, MAVS, cGAS, IRF3, and STING undergo LLPS to activate IFN signaling. Positive regulation of antiviral response is also present with TRIM25, NEMO, TFAM, HMGB, etc. Antiviral factors such as TRIM5a, MX1, NLRP3, NLRP6, etc., form liquid-like condensates and mediate antiviral immune response. At the same time, non-structural proteins of coronaviruses such as Nsp5 and Nsp1 participate in the negative regulation of antiviral responses.
How Coronaviruses Use LLPS to Their Advantage
-LLPS in Viral Replication
When a coronavirus infects a cell, its nucleocapsid (N) protein binds to the viral RNA, initiating LLPS. This process creates concentrated areas of viral components, which enhances viral replication and assembly. These droplets act like mini-factories, efficiently producing new virus particles.
-Evading the Immune Response
Coronaviruses have developed strategies to interfere with the host's innate immune response, which is the body's first line of defense against infections. The N proteins of coronaviruses can bind to host proteins involved in immune signaling, such as G3BP1. This binding disrupts the formation of stress granules, which are cellular structures that help limit viral replication. By preventing stress granule formation, coronaviruses can continue replicating unchecked.
-Interfering with Interferon Production
Interferons are proteins produced by infected cells to signal neighboring cells to mount an antiviral response. Coronaviruses can inhibit the production of interferons by disrupting the funct
ion of proteins like RIG-I and cGAS, which are crucial for detecting viral infections and initiating interferon production. By binding to these proteins, coronaviruses prevent them from triggering the necessary immune response, allowing the virus to spread more easily.
Potential Treatments Targeting LLPS
-Small Molecule Inhibitors
Scientists are exploring various small molecules that can disrupt the LLPS process of coronaviruses. For instance, compounds that interfere with the interaction between the N protein and RNA could prevent the formation of viral replication factories. Green tea polyphenol, gallocatechin gallate (GCG), has shown potential in disrupting N-RNA binding and inhibiting viral replication.
-Nucleoside Analogues
Nucleoside analogues mimic the building blocks of RNA and can interfere with viral replication. By competing with natural nucleotides, these analogues can be incorporated into the viral RNA, leading to errors in replication. ATP, a common cellular molecule, can also bind to the RNA-binding domains of N proteins, disrupting LLPS and hindering viral replication.
-Gene Regulation and Drug Screening
Research has identified several genes that regulate the formation of stress granules and immune responses. Drugs that modulate the expression of these genes could enhance the immune system's ability to combat viral infections. For example, imatinib and decitabine have shown promise in binding to G3BP1/2, proteins involved in stress granule formation, suggesting they could be repurposed to inhibit coronavirus replication.
The Road Ahead
-Challenges and Opportunities
While significant progress has been made in understanding the role of LLPS in coronavirus infections, there are still many unanswered questions. Most current studies are based on in vitro (test tube) experiments, and more research is needed to confirm these findings in living organisms. Developing specific tools to observe LLPS in real-time within infected cells will be crucial for advancing this field.
-Broad-Spectrum Antiviral Strategies
The insights gained from studying LLPS could lead to broad-spectrum antiviral strategies. Since LLPS is a common mechanism used by various viruses, targeting this process could provide a way to develop treatments for multiple viral infections. This approach could be especially valuable in preparing for future pandemics caused by novel viruses.
Conclusion
Understanding how coronaviruses exploit LLPS to evade the immune system opens new avenues for antiviral drug development. By targeting the mechanisms that allow these viruses to replicate and spread, scientists hope to create effective treatments not only for current coronavirus infections but also for other viral diseases. Continued research in this area promises to enhance our ability to combat viral outbreaks and protect public health.
The study findings were published in the peer reviewed journal: Biomolecules.
https://www.mdpi.com/2218-273X/14/7/748
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-evades-mucosal-immunity-via-orf8-protein-downregulating-polymeric-ig-receptor-pigr-expression
https://www.thailandmedical.news/news/autopsy-study-validates-that-sars-cov-2-causes-immune-dysregulation
