Scientists From Taiwan Find That CLEC2 Linked To SARS-CoV-2-Induced Thromboinflammation With CLEC2.Fc Emerging As A Potential Therapeutic Agent
COVID-19 News: CLEC2 - SARS-CoV-2-Induced Thromboinflammation May 23, 2023 1 year, 10 months, 3 weeks, 5 days, 11 hours, 31 minutes ago
COVID-19 News: The COVID-19 pandemic has brought unprecedented challenges to global health systems, with thromboinflammation emerging as a major cause of morbidity and mortality in affected patients. Thrombi and microangiopathy in vital organs have been observed during post-mortem examinations, and persistent microclots have been detected in both acute and long COVID-19 cases as reported in various past studies and
COVID-19 News coverages. However, the molecular mechanism behind SARS-CoV-2-induced thromboinflammation remains elusive.
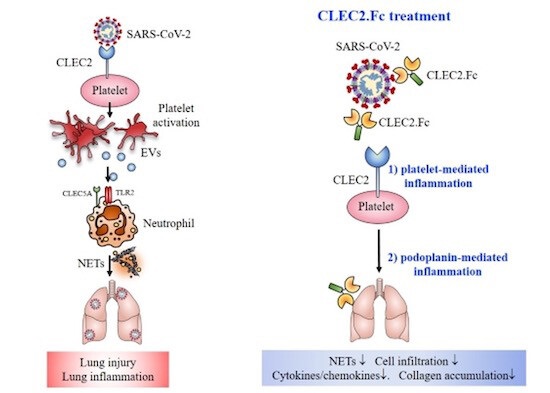 CLEC2 interacts with SARS-CoV-2 spike RBD and is critical for SARS-CoV-2-induced lung injury. In the presence of platelets, SARS-CoV-2 induces robust NET formation and cytokine release, while administration of CLEC2.Fc prevents platelet-mediated enhancement of NET formation and cytokine storm via blocking interactions between CLEC2 and SARS-CoV-2 spike protein.
CLEC2 interacts with SARS-CoV-2 spike RBD and is critical for SARS-CoV-2-induced lung injury. In the presence of platelets, SARS-CoV-2 induces robust NET formation and cytokine release, while administration of CLEC2.Fc prevents platelet-mediated enhancement of NET formation and cytokine storm via blocking interactions between CLEC2 and SARS-CoV-2 spike protein.
In a groundbreaking study, researchers from Taiwan have now identified CLEC2, a C-type lectin-like type II transmembrane receptor, as a key player in the development of SARS-CoV-2-induced thromboinflammation. Furthermore, they have discovered that a fusion protein called CLEC2.Fc could serve as a promising therapeutic agent to counteract this dangerous condition.
Understanding Thromboinflammation In COVID-19
Thromboinflammation, characterized by excessive blood clotting and inflammation, has been observed in severe cases of COVID-19. These microclots and thrombi can lead to organ damage and contribute to the severity of the disease. To unravel the mechanisms behind SARS-CoV-2-induced thromboinflammation, scientists focused on platelet activation and the formation of neutrophil extracellular traps (NETs), which play crucial roles in both clotting and inflammation processes.
The Role Of CLEC2 In Thromboinflammation
CLEC2, a receptor primarily expressed in platelets and alveolar macrophages, was found to interact directly with the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Unlike other pathogens, such as dengue virus, SARS-CoV-2 engages CLEC2 directly, activating platelets and enhancing the formation of aggregated NETs. These aggregated NETs, detached from surfaces, contribute to the formation of microemboli and microangiopathy observed in COVID-19 patients. By targeting CLEC2, it may be possible to inhibit platelet activation, NET formation, and subsequent thromboinflammation.
The Potential Of CLEC2.Fc As A Therapeutic Agent
In the study, the researchers administered CLEC2.Fc, a fusion protein consisting of the extracellular domain of CLEC2 and the Fc portion of human IgG1, to AAV-ACE2-infected mice. The results were promising, with CLEC2.Fc effectively inhibiting SARS-CoV-2-induced NET formation and thromboinflammation. These findings suggest that blocking the CLEC2-mediated signaling pathway could serve as a potential therapeutic strategy to alleviate thromboinflammation caused by SARS-CoV-2.
Implications And Future Directions
The discovery of CLEC2's involve
ment in SARS-CoV-2-induced thromboinflammation opens new avenues for the development of therapeutic interventions. By targeting CLEC2 with CLEC2.Fc, it may be possible to mitigate the risk of thrombotic complications and reduce the incidence of post-acute sequelae of COVID-19 (PASC) in patients.
Moreover, the study sheds light on the critical role of platelets and platelet-derived extracellular vesicles (EVs) in the pathogenesis of COVID-19-related microangiopathy. Understanding the interactions between platelets, EVs, and other immune cells could lead to the development of novel treatment strategies that target these pathways.
However, further research is needed to explore the potential off-target effects of CLEC2.Fc and optimize its binding affinity for various targets.
While CLEC2.Fc has demonstrated efficacy in reducing SARS-CoV-2-induced immunothrombosis, it is important to conduct further research to explore the potential off-target effects of this therapeutic agent.
Also, optimizing its binding affinity for specific targets will be crucial to enhance its therapeutic potential. One possible approach is the development of a CLEC2 mutant with a higher binding affinity for the Spike RBD of SARS-CoV-2. By engineering specific amino acid residues within the ligand-binding domain of CLEC2, scientists can create a variant that exhibits stronger and more selective binding to the intended targets. This would reduce the risk of off-target interactions and enhance the therapeutic efficacy of CLEC2.Fc.
Alternatively, researchers can explore the use of small molecule inhibitors that selectively block CLEC2 activation. By identifying and designing small molecules that specifically inhibit the interaction between the SARS-CoV-2 spike protein and CLEC2, it may be possible to prevent immunothrombosis without interfering with other physiological processes.
Preclinical and clinical studies should be conducted to assess the long-term safety, pharmacokinetics, biodistribution, and immunogenicity of CLEC2.Fc in relevant animal models and human subjects. These studies will provide valuable insights into the appropriate dosage, administration route, and treatment duration for CLEC2.Fc, ensuring its safe and effective use in patients.
Conclusions
The study's findings linking CLEC2 with SARS-CoV-2-induced thromboinflammation provide valuable insights into the pathogenesis of COVID-19. The discovery of CLEC2 as a novel pattern recognition receptor for SARS-CoV-2 highlights the critical role of platelets in immunothrombosis and microangiopathy. CLEC2.Fc, a fusion protein, shows potential as a therapeutic agent to inhibit SARS-CoV-2-induced thromboinflammation and reduce the risk of PASC. However, further research is needed to optimize the binding affinity of CLEC2.Fc, evaluate its safety and efficacy, and explore additional therapeutic targets within the C-type lectin family. These efforts will contribute to the development of effective interventions to combat the devastating consequences of COVID-19 and improve patient outcomes.
The study findings were published in the peer reviewed journal: EMBO Molecular Medicine.
https://www.embopress.org/doi/full/10.15252/emmm.202216351
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
