Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 03, 2024 1 year, 1 month, 5 hours, 35 minutes ago
COVID-19 News: The COVID-19 pandemic has sparked intensive research efforts to understand the virus's impact on various aspects of human health. In this
COVID-19 News report, a groundbreaking study is covered that was conducted by researchers from Peking Union Medical College Hospital, Peking Union Medical College, and the Chinese Academy of Medical Sciences, in collaboration with Geneplus-Shenzhen, China, that delved into the intricate world of cell-free DNA (cfDNA) methylation patterns. Their investigation explores the potential of cell-specific cfDNA markers in predicting and reflecting COVID-19 severity and outcomes, shedding light on the cellular dynamics associated with the disease.
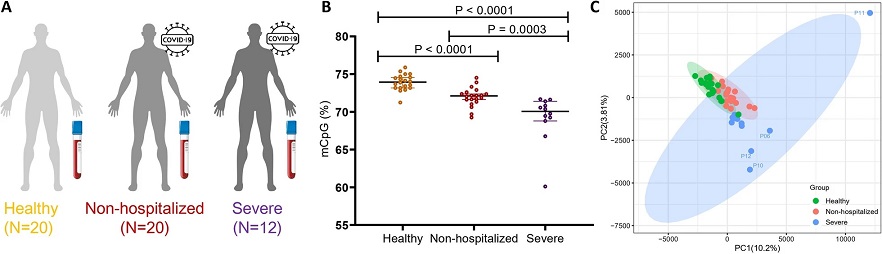 Cell-free DNA methylation reveals cell-specific tissue injury and correlates with disease severity and patient outcomes in COVID-19. Comparison of methylation levels among three cohorts. A Overview of three cohorts for DNA methylation profiles comparison. B The percentage of methylated CpG sites in three cohorts; C principal component analysis of CpG methylation levels
The Epigenetic Landscape: DNA Methylation as a Window
Cell-free DNA methylation reveals cell-specific tissue injury and correlates with disease severity and patient outcomes in COVID-19. Comparison of methylation levels among three cohorts. A Overview of three cohorts for DNA methylation profiles comparison. B The percentage of methylated CpG sites in three cohorts; C principal component analysis of CpG methylation levels
The Epigenetic Landscape: DNA Methylation as a Window
DNA methylation, a crucial epigenetic modification, plays a pivotal role in gene expression regulation and maintaining physiological functions. Recent revelations regarding distinct methylation patterns specific to cell types have opened avenues for understanding cell-specific responses in health and disease. Leveraging this breakthrough, the researchers embarked on a proof-of-concept study, utilizing COVID-19 as a model to investigate whether cell-specific cfDNA methylation signatures could serve as markers for disease severity and outcomes.
Methodology: Decoding the Methylation Patterns
To unravel the methylation intricacies associated with COVID-19, the team conducted whole-genome methylation sequencing of cfDNA from different cohorts, including healthy individuals, non-hospitalized COVID-19 cases, and severe COVID-19 patients admitted to the intensive care unit (ICU). The analysis focused on identifying differentially methylated regions (DMRs) and employed gene ontology pathway enrichment analyses to understand the locus-specific methylation differences between cohorts.
Results: Unveiling Global Methylation Dynamics
The study unearthed global reductions in cfDNA methylation levels in COVID-19 patients compared to healthy controls. Notably, severe COVID-19 patients exhibited a distinct cfDNA methylation signature with the identification of over 11,000 DMRs. Pathway enrichment analyses highlighted the involvement of these DMRs in immune response pathways, indicating a potential link between methylation patterns and the dysregulated immune response observed in severe cases.
Cell-Specific Insights: A Resolution at the Cellular Level
A novel algo
rithm was employed to estimate the tissue fraction of cfDNA derived from lung and immune cells at a cell-type resolution.
This approach revealed elevated levels of cfDNA from lung cells, particularly alveolar epithelial cells, bronchial epithelial cells, and lung endothelial cells, in COVID-19 patients compared to healthy controls. Furthermore, severe cases exhibited distinctive profiles, with higher levels of cfDNA from B cells, T cells, and granulocytes and lower levels from natural killer cells compared to non-hospitalized patients.
Key Biomarker: Alveolar Epithelial Cells
The researchers identified cfDNA derived from alveolar epithelial cells as a key biomarker, demonstrating optimal performance in differentiating COVID-19 severity levels, lung injury extents, Sequential Organ Failure Assessment (SOFA) scores, and in-hospital deaths. The robust discriminatory power of this biomarker, with an area under the receiver operating characteristic curve exceeding 0.9 in various assessments, highlights its potential clinical utility.
Discussion: Unraveling the Epigenetic Code of COVID-19
Drawing on previous studies, the authors compared their findings with existing literature, emphasizing the unique contribution of their cell-specific approach. The observed global reductions in cfDNA methylation levels and the distinct methylation profiles associated with severe cases underscore the potential of cfDNA methylation analysis in capturing disease-related abnormalities.
Insights into Immune Responses: cfDNA as an Immunomodulatory Indicator
The study also delved into the abnormal presence of immune cells, as reflected by cfDNA methylation signatures. Severe COVID-19 cases exhibited higher levels of cfDNA from various immune cells, indicating a potential link between immune cell death and disease severity. Notably, the proportion of cfDNA from natural killer cells was reduced in severe cases, suggesting a nuanced relationship between immune response and disease outcomes.
Tissue Injury Traced: The Alveolar Epithelial Cell Connection
A critical aspect of the study involved tracing tissue injury, particularly in lung cells, using cfDNA methylation. The researchers observed a correlation between the severity of lung injury and the tissue fraction of cfDNA derived from alveolar epithelial cells. This novel approach provides a unique window into the cellular dynamics of lung damage, offering insights into the progression of COVID-19.
Conclusion: Epigenetic Signatures as Disease Barcodes
In conclusion, this study unveils the distinct cfDNA methylation signature associated with severe COVID-19, providing a comprehensive understanding of the epigenetic landscape of the disease. The cell-specific resolution achieved through this analysis not only highlights the unique contributions of different cell types but also underscores the potential of cfDNA methylation as a diagnostic and prognostic tool. The identified biomarker, cfDNA derived from alveolar epithelial cells, emerges as a promising indicator of disease severity and clinical outcomes, paving the way for future applications in assessing tissue injuries in diseases characterized by multi-organ dysfunction. This research marks a significant step towards unraveling the epigenetic code of COVID-19 and harnessing its potential for precision medicine.
The study findings were published in the peer reviewed journal: Clinical Epigenetics.
https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-024-01645-7
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
