Nilhil Prasad Fact checked by:Thailand Medical News Team May 19, 2024 10 months, 3 weeks, 4 days, 14 hours, 1 minute ago
Medical News: Respiratory viral infections, including those caused by influenza and SARS-CoV-2, can wreak havoc on the lungs, leading to severe complications such as acute respiratory distress syndrome (ARDS). This condition, characterized by widespread inflammation and alveolar damage, has a mortality rate exceeding 40%. In the battle against these infections, inflammation serves a dual role: it is essential for recruiting immune cells to fight off the pathogens and repair the damaged tissue, but excessive inflammation can lead to further tissue damage and severe clinical outcomes. One of the critical players in this process appears to be a protein called SPARCL1. This
Medical News report explores how SPARCL1 exacerbates viral pneumonia, the underlying mechanisms, and the potential therapeutic implications.
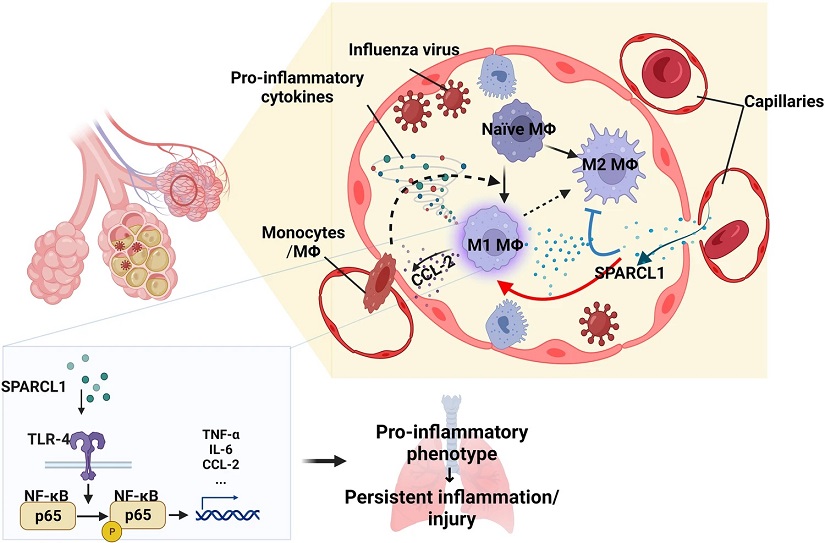 Illustration of the impact of SPARCL1 on the progression of influenza pneumonia.
In influenza pneumonia, SPARCL1 from endothelial cells enters the alveoli via damaged blood vessels, prompting alveolar macrophages to adopt a pro-inflammatory (M1-like) state. This transformation hinges on SPARCL1 inducing TLR-4/NF-κB signaling activation in alveolar macrophages, leading to increased expression of pro-inflammatory cytokines. Simultaneously, heightened CCL-2 attracts more monocytes and macrophages into the alveoli. These recruited macrophages, once again exposed to SPARCL1 within the alveoli, further adopt a pro-inflammatory phenotype. The cumulative effect intensifies the local inflammatory response, causing tissue damage and hindering lung repair.
The Role of Inflammation in Viral Pneumonia
Illustration of the impact of SPARCL1 on the progression of influenza pneumonia.
In influenza pneumonia, SPARCL1 from endothelial cells enters the alveoli via damaged blood vessels, prompting alveolar macrophages to adopt a pro-inflammatory (M1-like) state. This transformation hinges on SPARCL1 inducing TLR-4/NF-κB signaling activation in alveolar macrophages, leading to increased expression of pro-inflammatory cytokines. Simultaneously, heightened CCL-2 attracts more monocytes and macrophages into the alveoli. These recruited macrophages, once again exposed to SPARCL1 within the alveoli, further adopt a pro-inflammatory phenotype. The cumulative effect intensifies the local inflammatory response, causing tissue damage and hindering lung repair.
The Role of Inflammation in Viral Pneumonia
Inflammation is a crucial response to infection, with immune cells releasing various cytokines to recruit additional immune cells and promote tissue repair. However, when cytokine production becomes excessive, it can lead to a "cytokine storm," increasing vascular permeability, inducing further cell death, and exacerbating lung injury. This uncontrolled inflammation can manifest as sepsis, a life-threatening complication.
Macrophages, the most abundant immune cells in the lungs, are central to this inflammatory response. They can adopt different phenotypes, typically categorized as pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages. While M1 macrophages are essential for fighting infections, their prolonged activation can lead to tissue destruction. Conversely, M2 macrophages are involved in tissue repair and anti-inflammatory responses. Understanding how to control the balance between these macrophage phenotypes is crucial for managing viral pneumonia.
The Vascular Contribution to Inflammation
The endothelial cells (ECs) lining the pulmonary vasculature are not just passive barriers but active participants in lung function and immune responses. They release various signals that can influence macrophage behavior. One such signal is the matricellular protein SPARCL1, which has been implicated in o
ther inflammatory conditions but had not been previously studied in the context of viral pneumonia.
SPARCL1 in Viral Pneumonia
Researchers at the University of Pennsylvania and Massachusetts General Hospital found that SPARCL1 expression is significantly upregulated in pulmonary capillary endothelial cells during influenza-induced lung injury. This upregulation of SPARCL1 promotes a pro-inflammatory environment by inducing M1-like macrophages and related cytokines, worsening lung inflammation.
Mechanisms of SPARCL1 Action
SPARCL1 exerts its effects through Toll-like receptor 4 (TLR4) on macrophages. TLR4 is known for its role in activating the NF-κB pathway, a key regulator of inflammation. In vitro studies showed that SPARCL1 directly binds to TLR4, leading to the activation of NF-κB and the release of pro-inflammatory cytokines. In vivo, inhibiting TLR4 ameliorated the excessive inflammation caused by SPARCL1 overexpression in endothelial cells.
SPARCL1 and COVID-19
SPARCL1's role is not limited to influenza. The study also found increased SPARCL1 expression in lung endothelial cells from COVID-19 patients compared to healthy donors. Higher circulating SPARCL1 levels in the plasma were correlated with fatal outcomes in COVID-19 patients, suggesting that SPARCL1 could be a valuable prognostic biomarker and therapeutic target for COVID-19-induced pneumonia.
Endothelial Transcriptomics
To understand the dynamics of endothelial cells during viral lung injury, researchers isolated mouse lung ECs at various time points post-influenza infection and performed single-cell transcriptomic profiling. They identified six EC clusters, including two capillary EC clusters with distinct roles. One of these, termed immuneECs, showed high SPARCL1 expression during injury, indicating a potential role in regulating inflammation.
SPARCL1 Deletion and Overexpression
Using genetically modified mice, the researchers demonstrated that endothelial deletion of SPARCL1 reduced lung injury severity, improved recovery, and decreased pro-inflammatory cytokine levels. Conversely, overexpression of SPARCL1 in endothelial cells worsened pneumonia symptoms, increased pro-inflammatory cytokine levels, and reduced survival rates. These findings underscore the detrimental role of SPARCL1 in viral pneumonia.
Macrophage Polarization
Further experiments revealed that SPARCL1 promotes the polarization of macrophages towards the pro-inflammatory M1 phenotype. RNA sequencing of lung macrophages from SPARCL1-overexpressing mice showed significant upregulation of M1-associated genes and downregulation of M2-associated genes. This shift in macrophage polarization contributes to the persistence of inflammation and exacerbation of lung injury.
Therapeutic Implications
Given the critical role of SPARCL1 in driving inflammation through TLR4 signaling, targeting this pathway offers a promising therapeutic approach. Inhibition of TLR4 with the specific inhibitor TAK-242 significantly improved pneumonia symptoms in SPARCL1-overexpressing mice, suggesting that TLR4 inhibition could be beneficial for patients with high levels of SPARCL1. This therapeutic strategy could potentially be tailored to individuals based on their SPARCL1 levels, enabling personalized treatment approaches.
SPARCL1 as a Prognostic Biomarker
The study's findings also highlight the potential of SPARCL1 as a prognostic biomarker for viral pneumonia. Higher levels of SPARCL1 in the plasma were associated with worse outcomes in COVID-19 patients, suggesting that measuring SPARCL1 levels could help predict disease severity and guide treatment decisions.
Conclusion
SPARCL1 plays a significant role in exacerbating viral pneumonia by promoting a pro-inflammatory environment through TLR4 signaling. This discovery not only enhances our understanding of the mechanisms driving severe lung inflammation but also opens new avenues for therapeutic intervention. Targeting SPARCL1 or its downstream signaling pathways could help mitigate the harmful effects of excessive inflammation, improving outcomes for patients with viral pneumonia, including those affected by influenza and COVID-19. As research continues, the insights gained from this study may lead to more effective and personalized treatments for viral pneumonia, ultimately saving lives and reducing the burden of these devastating infections.
The study findings by researchers form University of Pennsylvania-USA and Massachusetts General Hospital and Harvard Medical School-USA were published in the peer reviewed journal: Nature Communiations.
https://www.nature.com/articles/s41467-024-48589-3
For more about Viral Pneumonia, keep on logging to Thailand
Medical News.
